Synthesis of gamma-decalactone from 1-octene and iodoacetic acid ethyl ester
(batch scale 10 mmol)
Substances
1-octen (bp 121 °C) 1.12 g (1.56 mL, 10.0 mmol)
iodoacetic acid ethyl ester (bp 73-74 °C/ 21 hPa) 2.78 g (1.54 mL, 13.0 mmol)
copper powder (finely powdered, > 230 mesh ASTM) 1.53 g (24.0 mmol)
tert-butyl methyl ether (bp 55 °C) 35 mL
Reaction
In a 50 mL two-neck flask with magnetic stir bar and a reflux condenser connected with a
protective gas piping 1.12 g (1.56 mL, 10.0 mmol) 1-octen are mixed with 2.78 g (1.54 mL,
13.0 mmol) iodoacetic acid ethyl ester and 1.53 g (24.0 mmol) copper powder under a
protective gas atmosphere. Afterwards the reaction mixture is stirred at 130 °C oil bath
temperature under protective gas for 2 hours under reflux. NOP http://www.oc-praktikum.de
Work up
The reaction mixture is cooled down to room temperature, diluted with 20 mL tert-butyl
methyl ether, stirred for 5 minutes and filtered off. The copper powder on the filter is washed
three times with 5 mL tert-butyl methyl ether each. Filtrate and wash solutions are combined,
the solvent is evaporated at the rotary evaporator. A liquid residue remains as crude product.
Crude yield: 1.5 g
The crude product is fractional distilled in a half-micro distillation apparatus under reduced
pressure.
Yield: 1.30 g ( 7.63 mmol, 77%); head temperature 85 °C (4.8·10-2 hPa, oil bath temperature
120 °C), colourless liquid; 20 nD = 1.4508

| 1H-NMR: gamma-Decalactone |
| 500 MHz, CDCl3 |
| delta [ppm] | mult. | atoms | assignment |
| 0.74 | t | 3 H | 10-H |
| 1.12-1.20 | m | 8 H | 6-H, 7-H, 8-H, 9-H |
| 1.39-1.77 | m | 3 H | 3-H, 5-Hb |
| 2.18 | m | 1 H | 5-Ha |
| 2.36 | dd | 2 H | 2-H |
| 4.33 | tt | 1 H | 4-H |
| 7.26 | | | CHCl3 |

| 13C-NMR: gamma-Decalactone |
| 125.7 MHz, CDCl3 |
| delta [ppm] | assignment |
| 13.9 | C10 |
| 22.4 | C9 |
| 25.0 | C5 |
| 27.9-29.0 | C6, C7, C8 |
| 31.5 | C3 |
| 35.4 | C2 |
| 80.9 | C4 |
| 177.1 | C1 (O-C(=O)-) |
| 76.5-77.5 | CDCl3 |
IR: gamma-Decalactone[Film, T%, cm-1][cm-1]assignment
Instruction (batch scale 100 mmol)
Equipment
100 mL two-neck flask, protective gas supply, reflux condenser, heatable magnetic stirrer,
magnetic stir bar, rotatory evaporator, high vacuum pump, distillation apparatus, oil bath
Substances
1-octen (bp 121 °C) 11.2 g (15.6 mL, 100 mmol)
iodoacetic acid ethyl ester (bp 73-74 °C/ 21 hPa) 27.8 g (15.4 mL, 130 mmol)
copper powder (finely powdered, > 230 mesh ASTM) 15.3 g (240 mmol)
tert-butyl methyl ether (bp 55 °C) 130 mL
NOP http://www.oc-praktikum.de
Reaction
In a 100 mL two-neck flask with magnetic stir bar and a reflux condenser connected with a
protective gas piping 11.2 g (15.6 mL, 100 mmol) 1-octen are mixed with 27.8 g (15.4 mL,
130 mmol) iodoacetic acid ethyl ester and 15.1 g (240 mmol) copper powder under a protectiv
gas atmosphere. Afterwards the reaction mixture is stirred at 130 °C oil bath temperature
under protective gas for 6 hours under reflux.
Work up
The reaction mixture is cooled down to room temperature, diluted with 30 mL tert-butyl
methyl ether, stirred for 5 minutes and filtered off. The copper powder on the filter is washed
4 times with 25 mL tert-butyl methyl ether each. Filtrate and wash solutions are combined,
the solvent is evaporated at the rotary evaporator. A liquid residue remains as crude product.
Crude yield: 15.9 g
The crude product is fractional distilled under reduced pressure.
Yield: 13.5 g (79.3 mmol, 79%); head temperature 70 °C (1.7·10-2 hPa, oil bath temperature
120 °C), colourless liquid; 20 nD = 1.4508
 | two-necked flask 50 mL | |  | protective gas piping |
 | reflux condenser | |  | heatable magnetic stirrer with magnetic stir bar |
 | rotary evaporator | |  | vacuum pump |
 | semi-micro distillation apparatus | |  | oil bath |

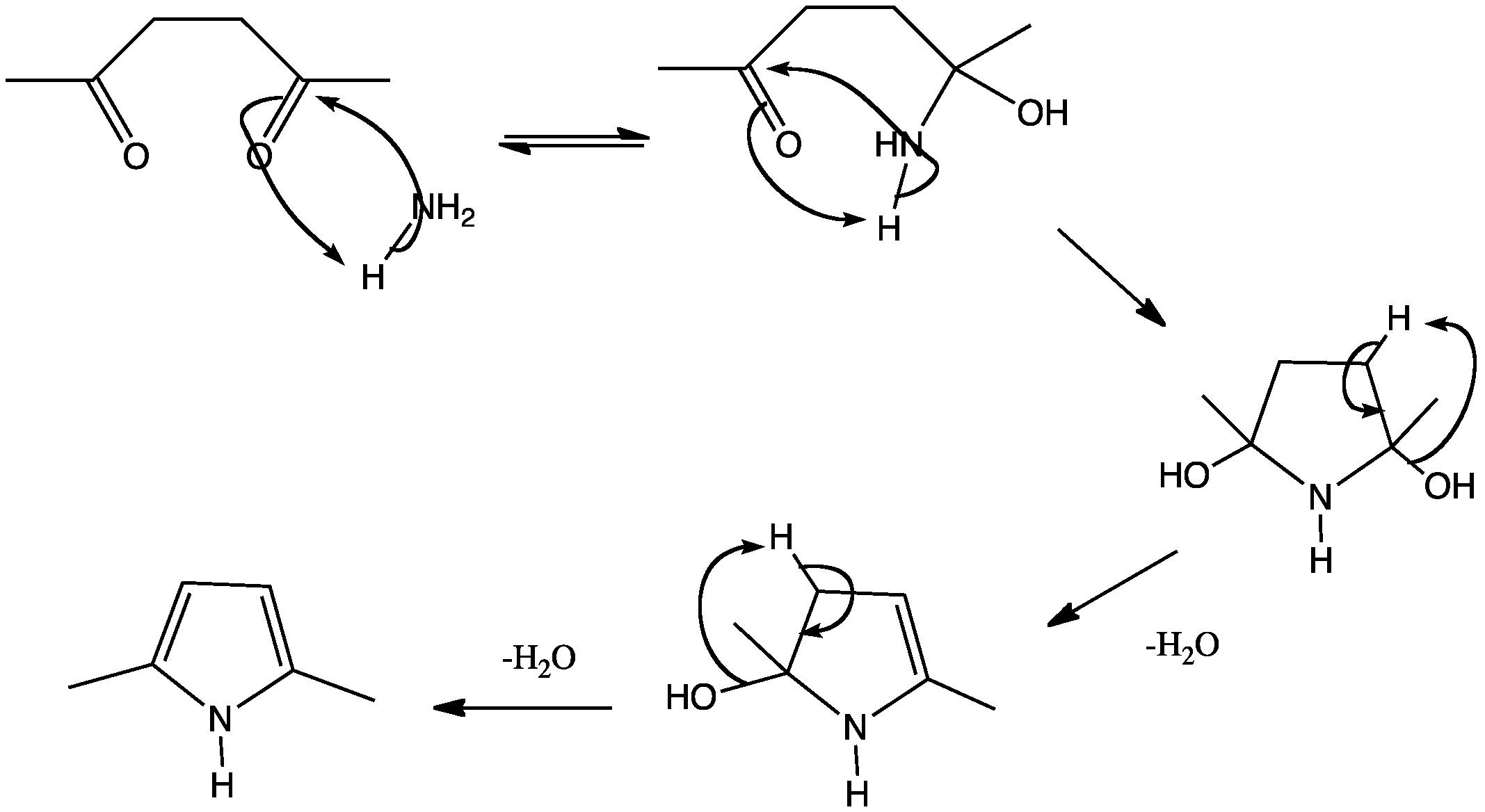
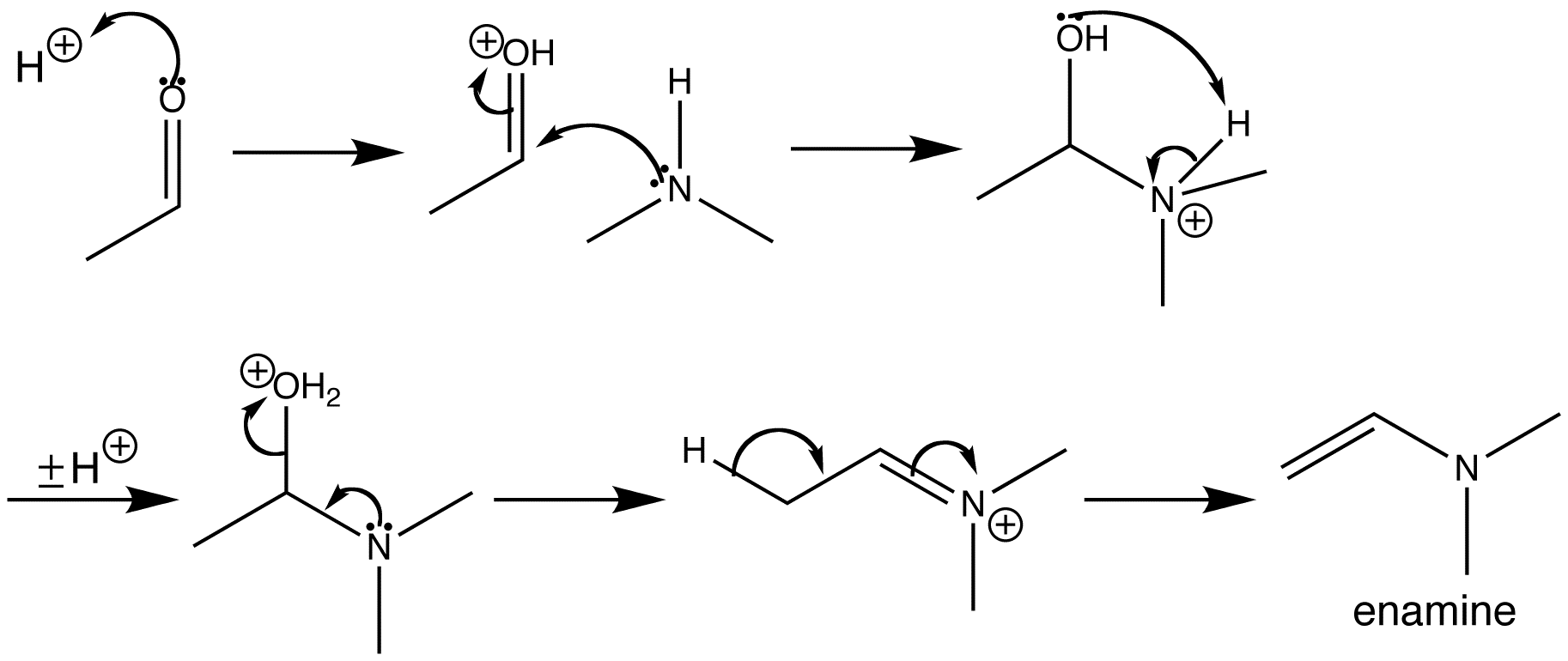
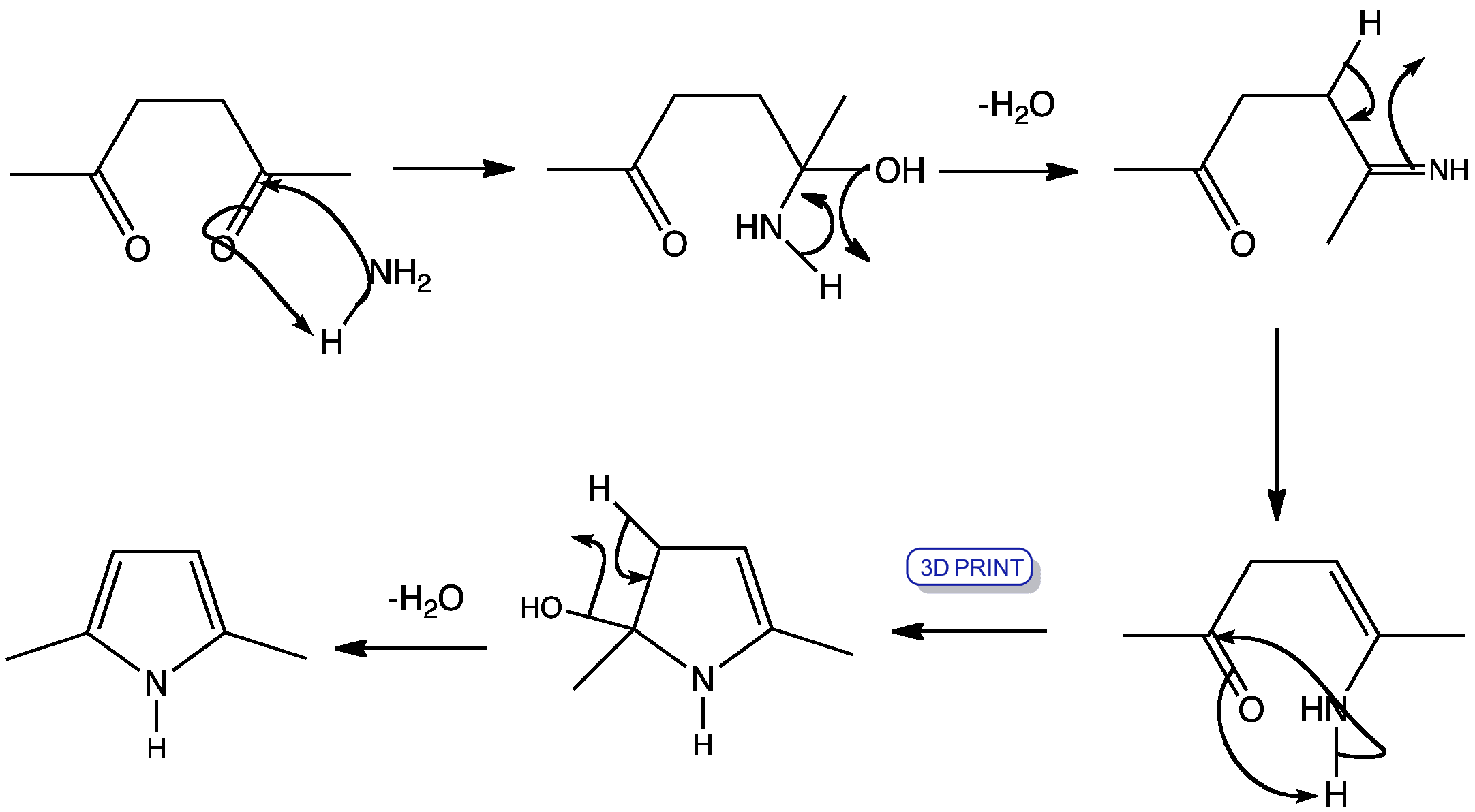
![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) ones as well as their further transformation to various heterocycles in a continuous-flow mode is presented. Furthermore, an extension of the simple flow configuration that allows for easy batch splitting and the generation of a heterocyclic library is described (see scheme).
ones as well as their further transformation to various heterocycles in a continuous-flow mode is presented. Furthermore, an extension of the simple flow configuration that allows for easy batch splitting and the generation of a heterocyclic library is described (see scheme).![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) ones as well as their further transformation to various heterocycles in a continuous-flow mode is presented. Furthermore, an extension of the simple flow configuration that allows for easy batch splitting and the generation of a heterocyclic library is described (see scheme).
ones as well as their further transformation to various heterocycles in a continuous-flow mode is presented. Furthermore, an extension of the simple flow configuration that allows for easy batch splitting and the generation of a heterocyclic library is described (see scheme).
 Methylene addends in fullerene electron acceptors increase
solar-cell efficiency
Methylene addends in fullerene electron acceptors increase
solar-cell efficiency
 Incorporation of a photosensitive azobenzene linker for
controllable carbohydrate orientation
Incorporation of a photosensitive azobenzene linker for
controllable carbohydrate orientation Halogen bonding drives the conversion from surface-confined
crystals to co-crystals without the use of solvent
Halogen bonding drives the conversion from surface-confined
crystals to co-crystals without the use of solvent