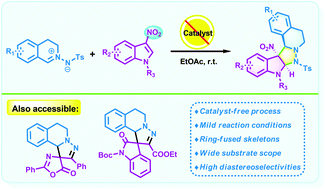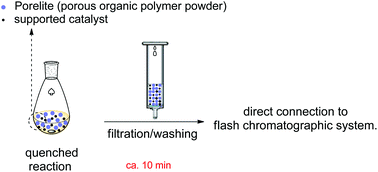
Simultaneous rapid reaction workup and catalyst recovery
By combining reaction work-up and catalyst recovery into a simple filtration procedure we have developed a substantially faster technique for organic synthesis. Our protocol eliminates the time-consuming conventional liquid–liquid extraction and is capable of parallelization and automation. Additionally, it requires only minimal amounts of solvent.
Simultaneous rapid reaction workup and catalyst recovery
*
Corresponding authors
a
Department of Chemistry, University of Louisville, Louisville, USA
E-mail: gb.hammond@louisville.edu
E-mail: gb.hammond@louisville.edu
b
College of Chemistry, Chemical Engineering and Biotechnology, Donghua University, 2999 North Renmin Lu, Shanghai 201620, China
E-mail: bo.xu@dhu.edu.cn
E-mail: bo.xu@dhu.edu.cn
Green Chem., 2016,18, 5769-5772
DOI: 10.1039/C6GC02448C
http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C6GC02448C?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
. General procedure for a reaction Step 1. Reaction setup. The reaction is conducted in the usual way with the supported catalyst. Porelite® (typically 1 mL for every 0.1 gram of product) is added to the reaction mixture under stirring, Step 2. Reaction quench and rigid solvent extraction. If needed, the reaction is quenched with a suitable aqueous solution (e.g. NaHCO3 solution). • If the solvent used in the reaction is water-miscible (eg., DMF, methanol, etc.), a minimum amount of water immiscible solvent (e.g. 3 mL ether for every 1 g of product) is added to help organic material become entrenched in Porelite. • If the reaction is conducted in a water immiscible solvent (e.g. toluene, DCM), no extra solvent is needed in most cases. The excess amount of solvent is removed by rotavapor or by nitrogen/air purging (no need to remove the water from the mixture). The reaction mixture is filtered to remove aqueous-soluble components (starting materials, by-products, etc.) and washed with water (or HCl or Na2CO3 solution to remove basic or acidic byproducts. Vacuum is applied to dry the filtrate for 2 minutes to remove any remaining aqueous and volatile solvents. (For automatic flash chromatographic separation, an empty loading cartridge can be used, which can be directly attached to the commercial system. For manual chromatographic separation, a regular Büchner filter can be used). Step 3. Sample loading to chromatographic system. • The loading cartridge can be directly attached to the commercial flash chromatographic system (e.g., CombiFlash Rf series). • For manual chromatographic separation, the polymer powder is loaded directly onto a manual flash silica gel column (dry loading).
Because the polymer pad may contain some trapped air, it is recommended to start with the least polar solvent (e.g., hexane) during chromatographic separation to remove the trapped air.
1-(biphenyl-4-yl)ethanone
1-(biphenyl-4-yl)ethanone
Han, W.; Liu, C.; Jin, Z.-L. Organic Letters 2007, 9, 4005-4007
////////