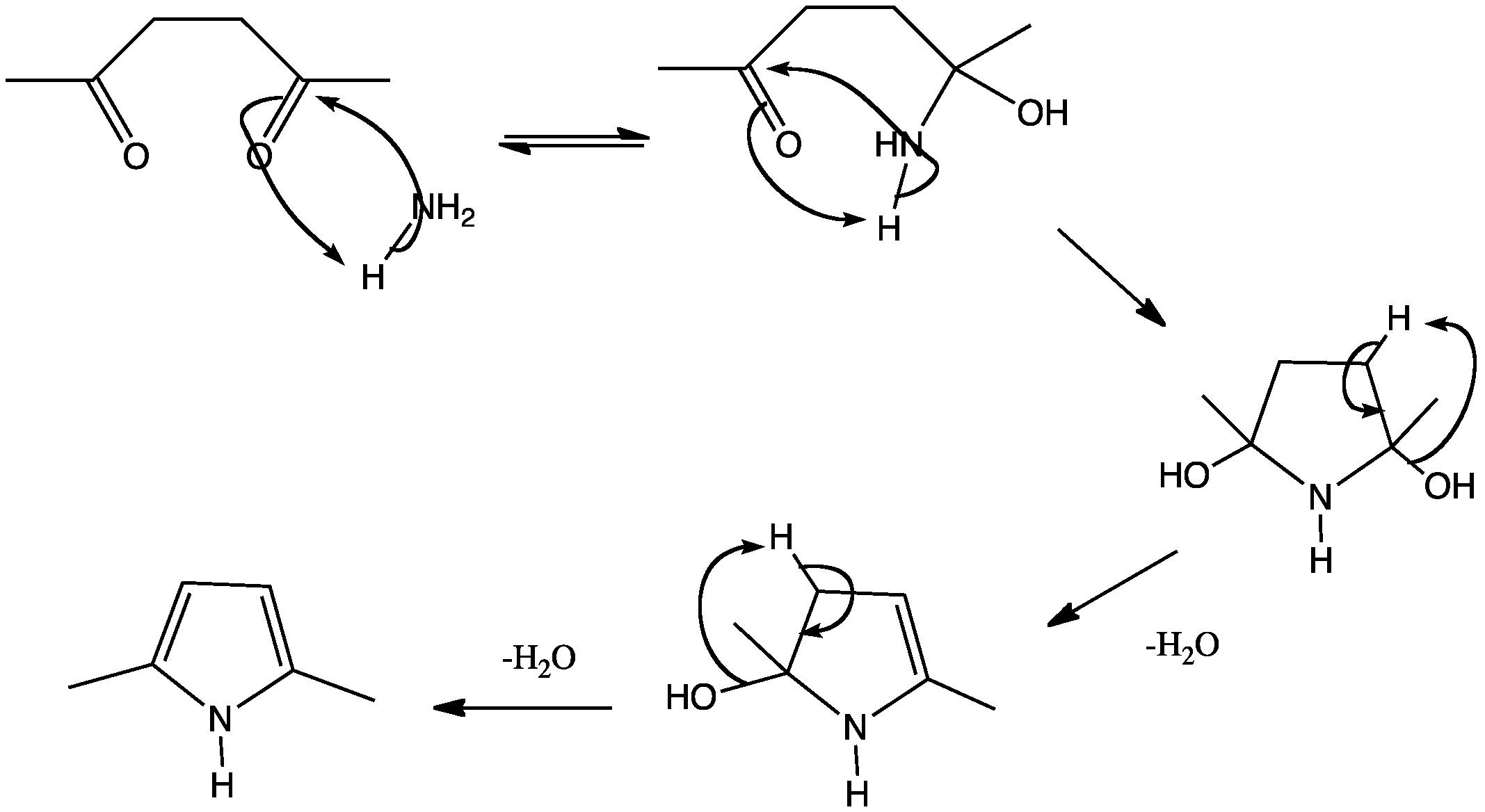
This shows the tradtional mechanism for forming a pyrrole ring using a 1,4-diketone and ammonia. The hydroxyl groups are removed as water in two separate dehydration steps, forming enamines in two of the intermediates.
A new mechanism, which does not require enamine formation, has been proposed based on a study using Density Functional Theory.
This shows the mechanism for pyrrole synthesis based on recent DFT study. Instead of the formation and cyclisation of a enamine intermediate, a hemiaminal intermediate in formed, followed by the consecutive dehydrations of the two hydroxyl groups. The dehydration steps may look familiar: it is just the formation of an enamine
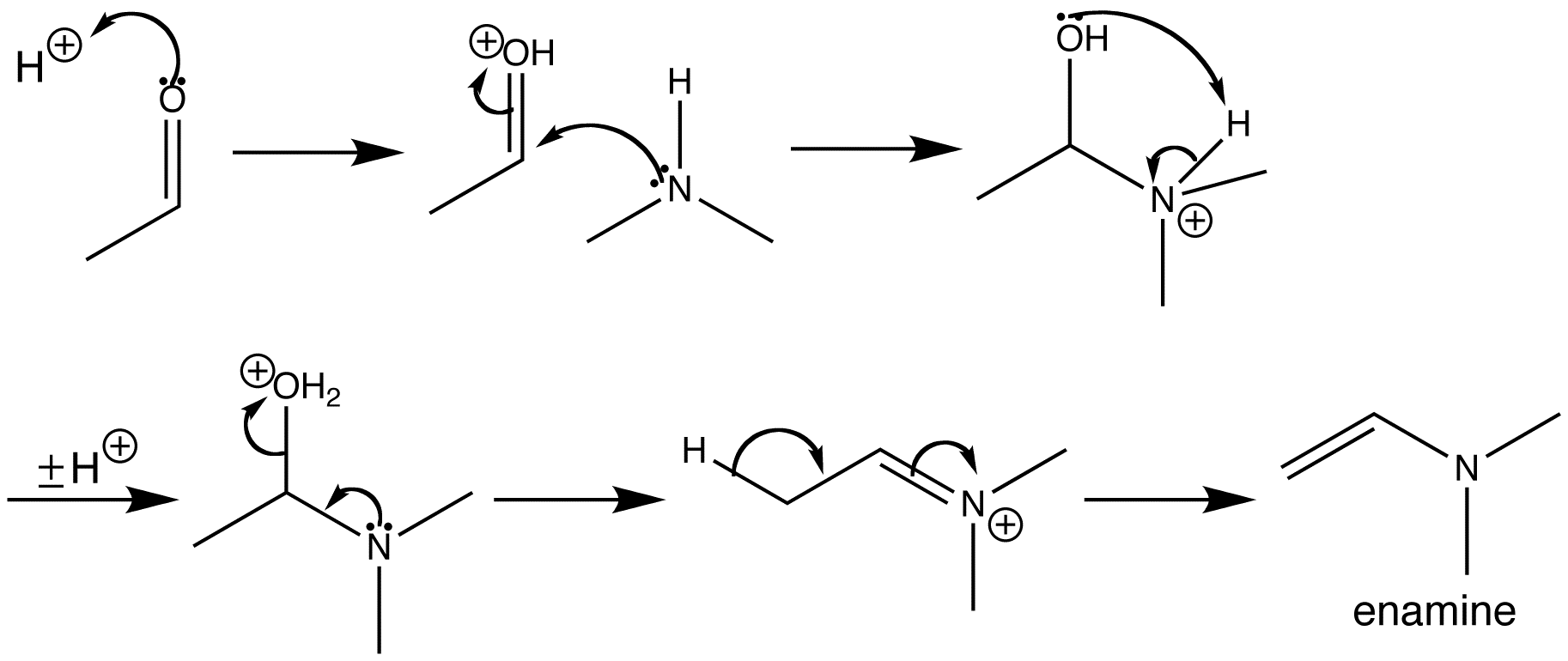
A more traditional approach to the mechanism, which appears in some text books and taught courses, can be found here.
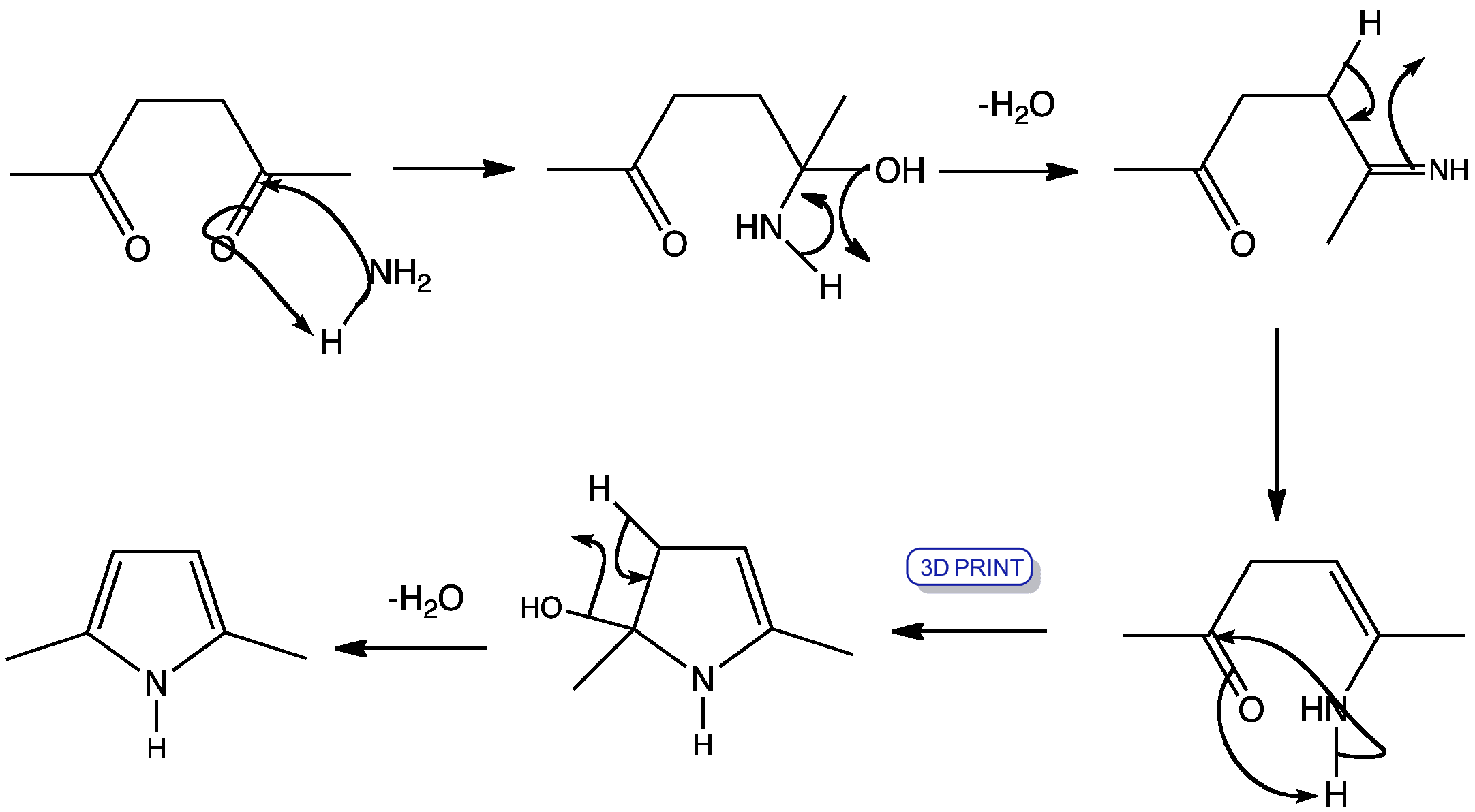
This mechanism was based on the paper: B. Mothana, R. J. Boyd, J. Mol. Struct.: THEOCHEM, 2007, 811, 97-107
No comments:
Post a Comment