
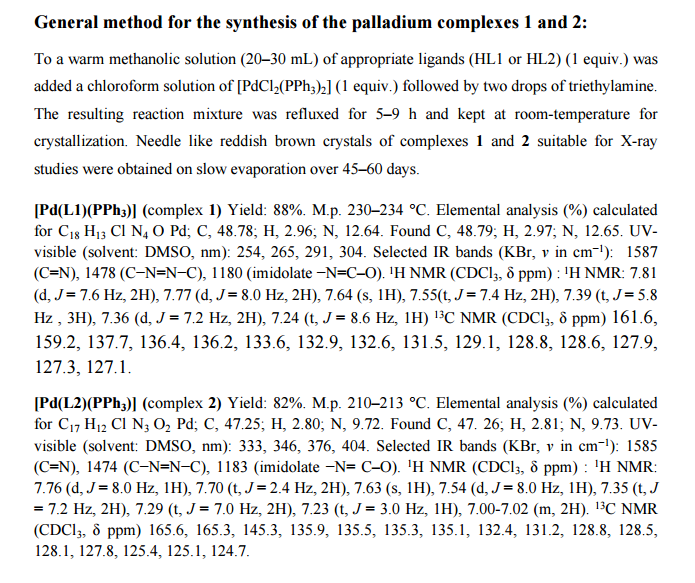
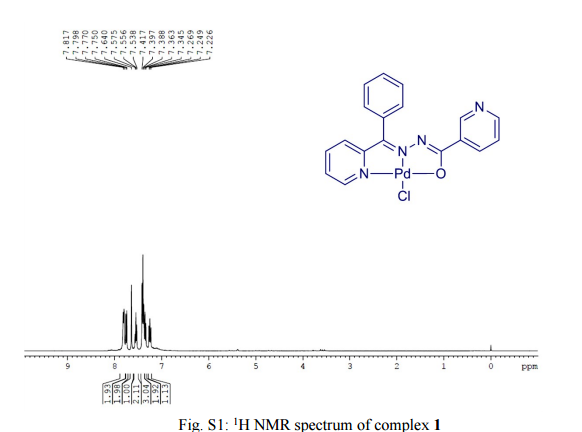
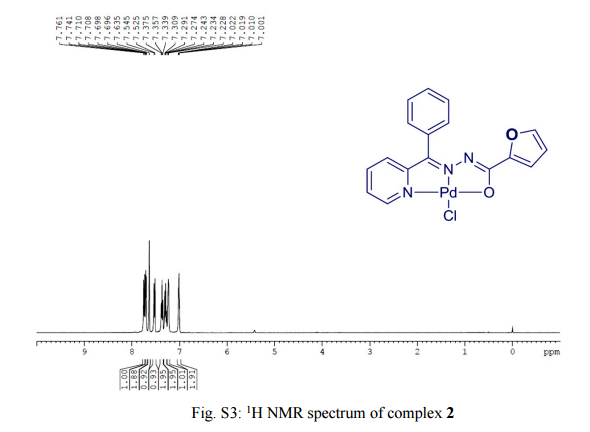
.
- Chemical Formula: C774H996N18O36Si12
- Molecular Weight: 11465.32
- Elemental Analysis: C, 81.08; H, 8.76; N, 2.20; O, 5.02; Si, 2.94
4-({2-[4-(2-{9-[4-(4-{2-[2,5-Bis(octyloxy)-4-(2-{4-[pentakis({4-[2-(4-{2-[4-(4-{2,7-bis[2-(4-{2-[(3-cyanopropyl)bis(propan-2-yl)silyl]ethynyl}-2,5-bis(octyloxy)phenyl)ethynyl]-9H-carbazol-9-yl}phenyl)phenyl]ethynyl}-2,5-bis(octyloxy)phenyl)ethynyl]phenyl})phenyl]phenyl}ethynyl)phenyl]ethynyl}phenyl)phenyl]-7-[2-(4-{2-[(3-cyanopropyl)bis(propan-2-yl)silyl]ethynyl}-2,5-bis(octyloxy)phenyl)ethynyl]-9H-carbazol-2-yl}ethynyl)-2,5-bis(octyloxy)phenyl]ethynyl}bis(propan-2-yl)silyl)butanenitrile
C774H996N18O36Si12
Name compound: 4-({2-[4-(2-{9-[4-(4-{2-[2,5-Bis(octyloxy)-4-(2-{4-[pentakis({4-[2-(4-{2-[4-(4-{2,7-bis[2-(4-{2-[(3-cyanopropyl)bis(propan-2-yl)silyl]ethynyl}-2,5-bis(octyloxy)phenyl)ethynyl]-9H-carbazol-9-yl}phenyl)phenyl]ethynyl}-2,5-bis(octyloxy)phenyl)ethynyl]phenyl})phenyl]phenyl}ethynyl)phenyl]ethynyl}phenyl)phenyl]-7-[2-(4-{2-[(3-cyanopropyl)bis(propan-2-yl)silyl]ethynyl}-2,5-bis(octyloxy)phenyl)ethynyl]-9H-carbazol-2-yl}ethynyl)-2,5-bis(octyloxy)phenyl]ethynyl}bis(propan-2-yl)silyl)butanenitrile.
https://www.instagram.com/p/BBMfA4bBkvZY9QhsJV6ff6khLjUE6F9MqBqRu40/
- Synthetic Procedure: See article for the definitive version of this procedure and for full experimental details.6 (19.0 mg, 14.7 µmol), (3.4 mg, 17.7 µmol), (4.0 mg, 4.4 µmol), and (6.0 mg, 29.5 µmol) were mixed in a microwave tube and sealed with a septum. In parallel, a solution of 5 (241.7 mg, 132.6 µmol) in
(2 mL) was purged with argon for 1 h. Then the latter was transferred
to the microwave tube and the mixture was heated at 120 °C for 16 min in
a microwave instrument (max. power: 300 W). After cooling to r.t., the
tube was opened and the reaction mixture was diluted with . The organic phase was washed with aq. (10 %) and brine. It was dried over and the solvent was removed under reduced pressure. The residue was purified by column chromatography (: = 65:35 ‑ 68:32), and 7 (126 mg, 10.9 µmol, 74 %) was obtained as a yellow solid. 1H NMR (500 MHz, , 298 K) δ [ppm] = 8.09 (d, 3JH,H = 8.2 Hz, 12 H), 7.87 (d, 3JH,H = 8.5 Hz, 12 H), 7.76 – 7.60 (m, 48 H), 7.55 – 7.46 (m, 12 H), 7.16 (d, 3JH,H = 8.0 Hz, 12 H), 7.03 (s, 6 H), 7.02 (s, 6 H), 6.96 (s, 12 H), 6.93 (s, 12 H), 6.86 (d, 3JH,H = 8.1 Hz, 12 H), 4.10 – 3.99 (m, 48 H), 3.99 – 3.90 (m, 24 H), 2.42 (t, 3JH,H = 7.0 Hz, 24 H), 1.95 – 1.72 (m, 96 H), 1.62 – 1.16 (m, 360 H), 1.15 – 0.97 (m, 168 H), 0.97 – 0.76 (m, 132 H); 1H NMR (500 MHz, CD2Cl2, 298 K) δ [ppm] = 8.14 (d, 3JH,H = 8.1 Hz, 12 H), 7.92 (d, 3JH,H = 8.5 Hz, 12 H), 7.79 – 7.66 (m, 36 H), 7.64 (s, 12 H), 7.54 – 7.44 (m, 12 H), 7.20 (d, 3JH,H
= 8.2 Hz, 12 H), 7.05 (s, 6 H), 7.02 (s, 6 H), 6.99 (s, 12 H), 6.97 –
6.93 (m, 24 H), 4.08 – 3.98 (m, 48 H), 3.98 – 3.92 (m, 24 H), 2.41 (t, 3JH,H = 7.0 Hz, 24 H), 1.96 – 1.71 (m, 96 H), 1.65 – 1.17 (m, 360 H), 1.17 – 0.99 (m, 168 H), 0.99 – 0.76 (m, 132 H); 13C NMR (126 MHz, ,
298 K) δ [ppm] = 154.78, 154.06, 153.94, 153.79, 141.60, 140.49,
140.41, 140.10, 136.79, 132.63, 131.73, 130.90, 128.94, 127.99, 127.36,
124.58, 123.55, 123.50, 121.54, 121.26, 120.86, 120.21, 118.04, 117.40,
117.28, 116.51, 114.96, 114.65, 114.07, 113.58, 113.47, 104.31, 96.51,
95.56, 94.91, 87.61, 86.62, 70.16, 70.04, 70.00, 69.56, 32.27, 32.25,
32.24, 32.18, 29.82, 29.79, 29.75, 29.73, 29.71, 29.68, 26.52, 26.42,
26.40, 23.14, 23.13, 23.09, 23.08, 21.70, 21.17, 18.65, 18.41, 14.63,
14.57, 14.52, 14.50, 12.20, 10.04; 13C NMR (126 MHz, CD2Cl2,
298 K) δ [ppm] = 154.88, 154.20, 154.11, 153.93, 141.87, 140.89,
140.63, 140.50, 140.40, 136.88, 132.68, 132.04, 130.83, 129.11, 128.15,
127.61, 124.49, 123.69, 123.50, 121.66, 121.44, 121.10, 120.32, 117.99,
117.47, 117.28, 116.70, 114.90, 114.62, 114.20, 113.84, 113.62, 104.31,
96.45, 95.91, 95.35, 94.94, 87.71, 86.85, 86.80, 70.22, 70.14, 69.82,
32.44, 32.35, 29.97, 29.93, 29.90, 29.88, 29.83, 29.83, 26.69, 26.67,
26.58, 26.54, 23.31, 23.29, 23.25, 23.24, 21.89, 21.22, 18.56, 18.32,
14.57, 14.50, 14.45, 14.43, 12.35, 10.14;MS (MALDI-TOF, ) (calcd. for C774H996N18O36Si12 monoisotopic: 11455.39, distr. max.: 11465.32): m/z11965.9 [M+2*DCTB]+, 11716.6 [M+DCTB]+, 11465.3 [M]+, 5856.8 [M+DCTB]2+, 5733.5 [M]2+; GPC (in vs. PS): Mp = 11580 g mol-1.
Soheil Zabihi
http://www.nature.com/nchem/journal/v5/n11/compound/nchem.1758_comp7.html
http://www.nature.com/nchem/journal/v5/n11/extref/nchem.1758-s1.pdf
http://www.nature.com/nchem/journal/v5/n11/extref/nchem.1758-s1.pdf
Affiliations
-
Kekulé-Institut für Organische Chemie und Biochemie der Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn, Germany
- A. Vikas Aggarwal,
- Alissa Idelson,
- Daniel Kalle,
- Stefan-S. Jester &
- Sigurd Höger
-
Department of Physics and Astronomy, University of Utah, Salt Lake City, Utah 84112, USA
- Alexander Thiessen &
- John M. Lupton
-
Institut für Experimentelle und Angewandte Physik, Universität Regensburg, Universitätsstrasse 31, D-93040 Regensburg, Germany
- Dominik Würsch,
- Thomas Stangl,
- Florian Steiner,
- Jan Vogelsang &
- John M. Lupton
///////////
SMILES: CCCCCCCCOC(C=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C(OCCCCCCCC)=C1)=C1C#CC2=CC3=C(C=C2)C4=C(C=C(C#CC5=C(OCCCCCCCC)C=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C(OCCCCCCCC)=C5)C=C4)N3C(C=C6)=CC=C6C(C=C7)=CC=C7C#CC(C=C8OCCCCCCCC)=C(OCCCCCCCC)C=C8C#CC(C=C9)=CC=C9C%10=C(C%11=CC=C(C#CC%12=C(OCCCCCCCC)C=C(C#CC%13=CC=C(C%14=CC=C(N%15C(C=C(C#CC%16=CC(OCCCCCCCC)=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C=C%16OCCCCCCCC)C=C%17)=C%17C%18=C%15C=C(C#CC%19=CC(OCCCCCCCC)=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C=C%19OCCCCCCCC)C=C%18)C=C%14)C=C%13)C(OCCCCCCCC)=C%12)C=C%11)C(C%20=CC=C(C#CC%21=C(OCCCCCCCC)C=C(C#CC%22=CC=C(C%23=CC=C(N%24C(C=C(C#CC%25=C(OCCCCCCCC)C=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C(OCCCCCCCC)=C%25)C=C%26)=C%26C%27=C%24C=C(C#CC%28=C(OCCCCCCCC)C=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C(OCCCCCCCC)=C%28)C=C%27)C=C%23)C=C%22)C(OCCCCCCCC)=C%21)C=C%20)=C(C%29=CC=C(C#CC%30=CC(OCCCCCCCC)=C(C#CC%31=CC=C(C%32=CC=C(N%33C(C=C(C#CC%34=C(OCCCCCCCC)C=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C(OCCCCCCCC)=C%34)C=C%35)=C%35C%36=C%33C=C(C#CC%37=C(OCCCCCCCC)C=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C(OCCCCCCCC)=C%37)C=C%36)C=C%32)C=C%31)C=C%30OCCCCCCCC)C=C%29)C(C%38=CC=C(C#CC%39=C(OCCCCCCCC)C=C(C#CC%40=CC=C(C%41=CC=C(N%42C(C=C(C#CC%43=CC(OCCCCCCCC)=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C=C%43OCCCCCCCC)C=C%44)=C%44C%45=C%42C=C(C#CC%46=CC(OCCCCCCCC)=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C=C%46OCCCCCCCC)C=C%45)C=C%41)C=C%40)C(OCCCCCCCC)=C%39)C=C%38)=C%10C%47=CC=C(C#CC%48=C(OCCCCCCCC)C=C(C#CC%49=CC=C(C%50=CC=C(N%51C(C=C(C#CC%52=C(OCCCCCCCC)C=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C(OCCCCCCCC)=C%52)C=C%53)=C%53C%54=C%51C=C(C#CC%55=CC(OCCCCCCCC)=C(C#C[Si](C(C)C)(C(C)C)CCCC#N)C=C%55OCCCCCCCC)C=C%54)C=C%50)C=C%49)C(OCCCCCCCC)=C%48)C=C%47










 .
.