Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02920A, Communication
DOI: 10.1039/C5GC02920A, Communication
Anuja Nagendiran, Henrik Sorensen, Magnus J. Johansson, Cheuk-Wai Tai, Jan-E. Backvall
A continuous-flow approach towards the selective nanopalladium-catalyzed hydrogenation of the olefinic bond in various Michael acceptors, which could lead to a greener and more sustainable process, has been developed.
A continuous-flow approach towards the selective nanopalladium-catalyzed hydrogenation of the olefinic bond in various Michael acceptors, which could lead to a greener and more sustainable process, has been developed.
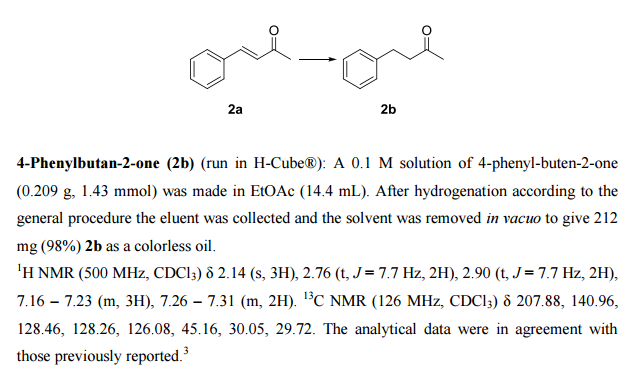
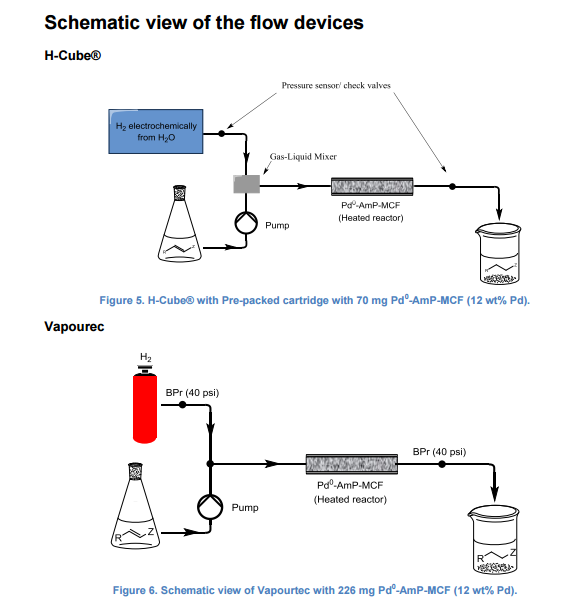
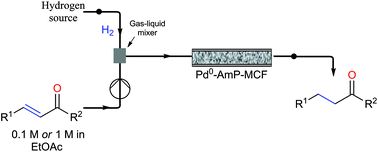
A continuous-flow approach towards the selective nanopalladium-catalyzed hydrogenation of the olefinic bond in various Michael acceptors, which could lead to a greener and more sustainable process, has been developed. The nanopalladium is supported on aminofunctionalized mesocellular foam. Both aromatic and aliphatic substrates, covering a variation of functional groups such as acids, aldehydes, esters, ketones, and nitriles were selectively hydrogenated in high to excellent yields using two different flow-devices (H-Cube® and Vapourtec). The catalyst was able to hydrogenate cinnamaldehyde continuously for 24 h (in total hydrogenating 19 g cinnanmaldehyde using 70 mg of catalyst in the H-cube®) without showing any significant decrease in activity or selectivity. Furthermore, the metal leaching of the catalyst was found to be very low (ppb amounts) in the two flow devices
3 Gottlieb, H. E.; Kotlyar, V; Nudelman, A. J. Org. Chem. 1997, 62, 7512-7515.
Nanopalladium-catalyzed conjugate reduction of Michael acceptors – application in flow
*Corresponding authors
aDepartment of Organic Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91 Stockholm, Sweden
E-mail: jeb@organ.su.se
E-mail: jeb@organ.su.se
bBerzelii Centre EXSELENT on Porous Materials, Arrhenius Laboratory, Stockholm University, SE-106 91 Stockholm, Sweden
cAstraZeneca R&D, Innovative Medicines, Cardiovascular and Metabolic Disorders, Medicinal Chemistry, Pepparedsleden 1, SE-431 83 Mölndal, Sweden
dDepartment of Materials and Environmental Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91, Stockholm, Sweden
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02920A
///////