Improving the efficiency of the Diels-Alder process by using flow chemistry and zeolite catalysis
Improving the efficiency of the Diels–Alder process by using flow chemistry and zeolite catalysis
*Corresponding authors
a
SynBioC Research Group, Department of Sustainable Organic Chemistry and Technology, Faculty of Bioscience Engineering, Ghent University, Coupure Links 653, 9000 Ghent, Belgium
E-mail: chris.stevens@ugent.be
E-mail: chris.stevens@ugent.be
b
VITO, Vlaamse Instelling voor Technologisch Onderzoek, Boeretang 200, 2400 Mol, Belgium
c
Laboratory for Chemical Technology, Department of Chemical Engineering and Technical Chemistry, Faculty of Engineering and Architecture, Ghent University, Technologiepark 914, 9052 Ghent, Belgium
Green Chem., 2017,19, 237-248
DOI: 10.1039/C6GC02334G
http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C6GC02334G?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
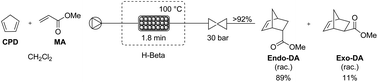
The industrial application of the Diels–Alder reaction for the atom-efficient synthesis of (hetero)cyclic compounds constitutes an important challenge. Safety and purity concerns, related to the instability of the polymerization prone diene and/or dienophile, limit the scalability of the production capacity of Diels–Alder products in a batch mode. To tackle these problems, the use of a high-pressure continuous microreactor process was considered. In order to increase the yields and the selectivity towards the endo-isomer, commercially available zeolites were used as a heterogeneous catalyst in a microscale packed bed reactor. As a result, a high conversion (≥95%) and endo-selectivity (89
 :
: 11) were reached for the reaction of cyclopentadiene and methyl acrylate, using a 1
11) were reached for the reaction of cyclopentadiene and methyl acrylate, using a 1 :
: 1 stoichiometry. A throughput of 0.87 g h−1during at least 7 h was reached, corresponding to a 3.5 times higher catalytic productivity and a 14 times higher production of Diels–Alder adducts in comparison to the heterogeneous lab-scale batch process. Catalyst deactivation was hardly observed within this time frame. Moreover, complete regeneration of the zeolite was demonstrated using a straightforward calcination procedure.
1 stoichiometry. A throughput of 0.87 g h−1during at least 7 h was reached, corresponding to a 3.5 times higher catalytic productivity and a 14 times higher production of Diels–Alder adducts in comparison to the heterogeneous lab-scale batch process. Catalyst deactivation was hardly observed within this time frame. Moreover, complete regeneration of the zeolite was demonstrated using a straightforward calcination procedure.//////Diels-Alder, flow chemistry, zeolite catalysis