
The
Darzens reaction (also known as the
Darzens condensation or
glycidic ester condensation) is the
chemical reaction of a
ketone or
aldehyde with an α-
haloester in the presence of
base to form an α,β-
epoxy ester, also called a "glycidic ester".
[1][2] This reaction was discovered by the organic chemist
Auguste George Darzens in 1904.
[3]

The reaction process begins when a strong base is used to form a carbanion at the halogenated position. Because of the ester, this carbanion is a resonance-stabilized enolate, which makes it relatively easy to form. This nucleophilic structure attacks another carbonyl component, forming a new carbon–carbon bond. These first two steps are similar to a base-catalyzed aldol reaction. The oxygen anion in this aldol-like product then does an intramolecular SN2 attack on the formerly-nucleophilic halide-bearing position, displacing the halide to form an epoxide.[4] This reaction sequence is thus a condensation reaction, since there is a net loss of HCl when the two reactant molecules join.Reaction mechanism

The primary role of the ester is to enable the initial deprotonation
to occur, and other carbonyl functional groups can be used instead. If
the starting material is an α-halo
amide, the product is an α,β-epoxy amide.
[5] If an α-halo ketone is used, the product is an α,β-epoxy ketone.
[4]
Any sufficiently strong base can be used for the initial deprotonation. However, if the starting material is an ester, the
alkoxide corresponding to the ester side-chain is commonly in order to prevent complications due to potential
acyl exchange
side reactions.
Stereochemistry
Depending on the specific structures involved, the epoxide may exist in
cis and trans forms. A specific reaction may give only
cis, only
trans, or a mixture of the two. The specific
stereochemical outcome of the reaction is affected by several aspects of the intermediate steps in the sequence.
The initial stereochemistry of the reaction sequence is established in the step where the carbanion attacks the carbonyl. Two
sp3 (tetrahedral) carbons are created at this stage, which allows two different
diastereomeric possibilities of the
halohydrin intermediate. The most likely result is due to
chemical kinetics: whichever product is easier and faster to form will be the major product of this reaction. The subsequent S
N2 reaction step proceeds with stereochemical inversion, so the
cis or
trans
form of the epoxide is controlled by the kinetics of an intermediate
step. Alternately, the halohydrin can epimerize due to the basic nature
of the reaction conditions prior to the S
N2 reaction. In this case, the initially formed diastereomer can convert to a different one. This is an
equilibrium process, so the
cis or
trans form of the epoxide is controlled by
chemical thermodynamics--the product resulting from the more stable diastereomer, regardless of which one was the kinetic result.
[5]
Alternative reactions
Glycidic esters can also be obtained via
nucleophilic epoxidation of an
α,β-unsaturated ester,
but that approach requires synthesis of the alkene substrate first
whereas the Darzens condensation allows formation of the carbon–carbon
connectivity and epoxide ring in a single reaction.
Subsequent reactions
The product of the Darzens reaction can be reacted further to form various types of compounds.
Hydrolysis of the ester can lead to
decarboxylation, which triggers a
rearrangement of the epoxide into a carbonyl (
4). Alternately, other epoxide rearrangements can be induced to form other structures.
-
References
- Darzens, G. (1905). Compt. Rend. 141: 766.
- Darzens, G. (1906). Compt. Rend. 142: 214.
- Darzens, G. (1904). Compt. Rend. 139: 1214.
- Jie Jack Li (2006). "Darzens glycidic ester condensation". Name Reactions (3rd. expanded ed.). Springer-Verlag. pp. 183–184. doi:10.1007/3-540-30031-7.
- Tung,
C. C.; Speziale, A. J.; Frazier, H. W. (1963). "The Darzens
Condensation. II. Reaction of Chloroacetamides with Aromatic Aldehydes".
The Journal of Organic Chemistry 28 (6): 1514. doi:10.1021/jo01041a018. edit
Review articles
- Newman, M. S.; Magerlein, B. J. (1949). Org. React. 5: 413.
- Ballester, M. (1955). "Mechanisms of The Darzens and Related Condensations Manuel Ballester". Chem. Rev. 55 (2): 283. doi:10.1021/cr50002a002.
- Rosen, T. (1991). Comp. Org. Syn. 2: 409–439.


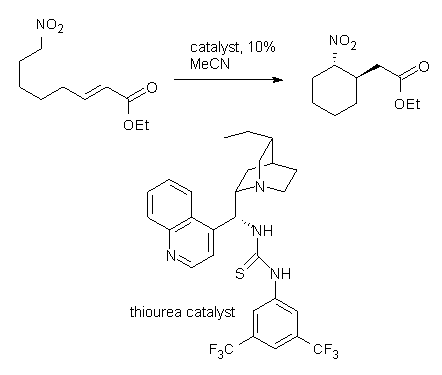





 2-benzoxopinones were synthesized by a dual activation strategy, involving N-heterocyclic carbene catalysis and a second Lewis base
2-benzoxopinones were synthesized by a dual activation strategy, involving N-heterocyclic carbene catalysis and a second Lewis base
















 BASF plans production of butanediol from renewable feedstock using Genomatica technology
BASF plans production of butanediol from renewable feedstock using Genomatica technology Evonik has started basic engineering for a production plant for precipitated silica in Brazil
Evonik has started basic engineering for a production plant for precipitated silica in Brazil