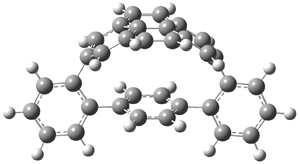
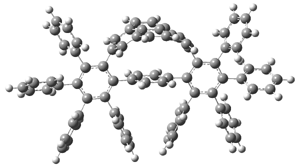
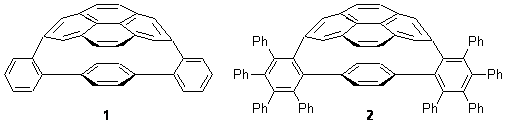
The novel cyclophanes 1 and 2 have now been synthesized.1 An interesting question is whether the bent pyrenes portion of the two molecules remains aromatic. The bending angles is 93.8° in 1and 95.8° in 2. This distortion is readily apparent in Figure 1, which presents their B3LYP/6-311G(d,p) optimized geometries. NICS computations were used to assess the aromaticity of the pyrene portion. The central rings of pyrene have NICS(0) = -4.4 ppm. The corresponding values in1 and 2 are -4.5 ppm. The apical rings of pyrene have NICS(0)= -11.9 ppm, while the value is -11.1 ppm in 1 and -11.0 ppm in 2. These calculations indicate that the molecule retains much of the aromaticity of the parent pyrene despite the significant out-of-plane distortions.
|
1
|
2
|
References
(1) Zhang, B.; Manning, G. P.; Dobrowolski, M. A.; Cyranski, M. K.; Bodwell, G. J., "Nonplanar Aromatic Compounds. 9. Synthesis, Structure, and Aromaticity of 1:2,13:14-Dibenzo[2]paracyclo[2](2,7)-pyrenophane-1,13-diene," Org. Lett., 2008, 10, 273-276, DOI: 10.1021/ol702703b.