Aerobic flow oxidation of alcohols in water catalyzed by platinum nanoparticles dispersed in an amphiphilic polymer

Osako, T.; Toriia, K.; Uozumi, Y.; Aerobic flow oxidation of alcohols in water catalyzed by platinum nanoparticles dispersed in an amphiphilic polymer; RSC Adv.; 2015; 5; 2647-2654
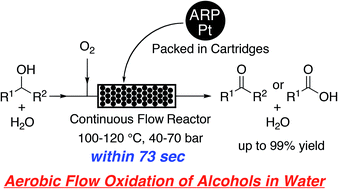
We have developed a technique for the aqueous aerobic flow oxidation of alcohols in a continuous-flow reactor containing platinum nanoparticles dispersed on an amphiphilic polystyrene–poly(ethylene glycol) resin (ARP-Pt). Various primary and secondary alcohols including aliphatic, aromatic and heteroaromatic alcohols were efficiently oxidized within 73 seconds in a flowing aqueous system at 100–120 °C under 40–70 bar of the system pressure to give the corresponding carboxylic acids and ketones, respectively, in up to 99% yield. Benzaldehydes could be also prepared selectively from benzyl alcohols by conducting the flow oxidation under the standard conditions in the presence of triethylamine. Moreover, a practical gram-scale synthesis of surfactants was realized in the aqueous aerobic continuous flow oxidation for 36–116 hours. This aerobic flow oxidation system provides a safe, clean, green, rapid and efficient practical method for oxidizing alcohols.
Paper
Aerobic flow oxidation of alcohols in water catalyzed by platinum nanoparticles dispersed in an amphiphilic polymer
*
Corresponding authors
a
Institute for Molecular Science (IMS) Myodaiji, Okazaki, Japan
E-mail: uo@ims.ac.jp
E-mail: uo@ims.ac.jp
b
RIKEN, Wako, Japan
RSC Adv., 2015,5, 2647-2654
DOI: 10.1039/C4RA14947E
 Yasuhiro Uozumi
Yasuhiro Uozumi
D.Pharm.
Okazaki, Japan

 Reusable single-site Ru catalyst on mesoporous silica for
selective C-H oxidation of hydrocarbons
Reusable single-site Ru catalyst on mesoporous silica for
selective C-H oxidation of hydrocarbons
![Synthetic Scheme for 1,2,4-triazolo[3,4-b][1,3,4]thiadiazine bearing substituted ...](http://ars.els-cdn.com/content/image/1-s2.0-S187853521500218X-gr2.jpg)
 C and C
C and C S
S