Green Chem., 2014, Advance Article
DOI: 10.1039/C4GC00003J, Paper
DOI: 10.1039/C4GC00003J, Paper
Jian-An Jiang, Cheng Chen, Ying Guo, Dao-Hua Liao, Xian-Dao Pan, Ya-Fei Ji
A highly efficient three-step approach to vanillin has been developed starting from 4-cresol.
A highly efficient three-step approach to vanillin has been developed starting from 4-cresol.
A highly efficient approach to the famous flavor and fragrance compound vanillin has been developed starting from 4-cresol with the attention focused on improving the sustainability of all the reactions. The approach involves a three-step sequence of the quasi-quantitative selective clean oxybromination of 4-cresol, the high-yield selective aerobic oxidation of 2-bromo-4-cresol, and the quantitative methoxylation of 3-bromo-4-hydroxybenzaldehyde with the recovery of pure methanol. Herein, the pivotal oxidation and methoxylation reactions are logically investigated and developed into two concise methodologies. As a green alternative, the approach holds significant value for the sustainable manufacturing of vanillin.
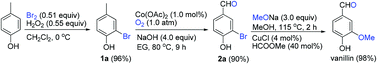
 ChemDraw is a leading chemistry molecules drawing software. ChemDraw is easy to use and best software for drawing a molecule structures and finding stereochemistry of the molecules.
ChemDraw is a leading chemistry molecules drawing software. ChemDraw is easy to use and best software for drawing a molecule structures and finding stereochemistry of the molecules.


 Parkinson's is a neurodegenerative disease
Parkinson's is a neurodegenerative disease
