Lead tetraacetate oxidation of N-aminophthalimide in the presence of azo compounds affords phthalimidoazimines, a new class of 1,3-dipolar species [2,3,7].
Azo-alkenes (a,b-unsaturated azo compounds) [8] containing both functional groups aroused our interest to investigate the chemoselectivity of this reaction. To this goal, N-aminophthalimide was oxidized with lead tetraacetate in the presence of several azo-alkenes.
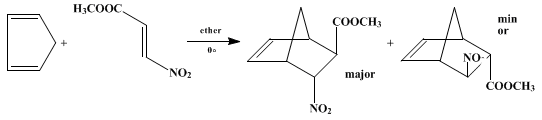
Scheme 1 Oxidation of 1,1-disubstituted hydrazines in the presence of olefins and azo compounds: N-aminoaziridines and azimines

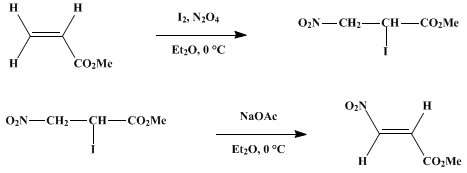
Scheme 2 Azo-alkenes; syntheses

Scheme 3 (E)-b-Phenylazo-stilbene, N-aminophthalimide, lead tetraacetate: 2-phenyl-3-phenylazo-1-phthalimidoaziridine

Scheme 4 (E)-1-phenylazo-1-cyclohexene, N-aminophthalimide, lead tetraacetate: syn-2-phenylazo-1-phthalimidoperhydrocyclohexa[b]aziridine

Scheme 5 (E)-1-phenylazo-1-cyclopentene, N-aminophthalimide, lead tetraacetate: 2-phenyl-2,4,5,6-tetrahydrocyclopenta[d][1,2,3]triazole

Scheme 6 (E)-2-phenylazo-propene, N-aminophthalimide, lead tetraacetate: 4-methyl-2-phenyl-1,2,3-triazole and phthalimide and
(2-phenylhydrazonopropylideneamino)phthalimide [via ene-azimine?]

Scheme 7 3,3,5-Trimethyl-3H-pyrazole, N-aminophthalimide, lead tetraacetate: (E)- and (Z)-3,3,5-trimethyl-3H-pyrazolium-2-(phthalimido)imide and (Z)-3,3,5-trimethyl-3H-pyrazolium-1-(phthalimido)imide

References
- B.V. Ioffe, M.A. Kuznetsov, A.A. Potekhin, "Chemistry of the Organic Hydrazine Derivatives" Khimiya, Leningrad, 1979, p. 224.
- R.S. Atkinson in "Azides and Nitrenes. Reactivity and Utility" ed. E.F.V. Scriven, Academic Press, New York, 1984, p. 247.
- M.A. Kuznetsov, B.V. Ioffe, Uspekhi Khimii, 1989, 58, 1271; Russ. Chem. Rev., 1989, 58, 732.
- R.S. Atkinson, B.J. Kelly, J. Chem. Soc., Chem. Commun. 1987, 1362.
- D.W. Jones, M. Thorton-Pett, J. Chem. Soc., Perkin Trans. 1 1995, 809.
- R.S. Atkinson, E. Barker, J. Chem. Soc., Chem. Commun., 1995, 819.
- A.A. Suvorov, M.A. Kuznetsov, Uspekhi Khimii, 1987, 56, 1324; Russ. Chem. Rev. 1987, 56, 756.
- J.G. Schantl in Houben-Weyl, "Methoden der Organischen Chemie" vol. E15, Georg Thieme, Stuttgart-New York, 1993, 909.