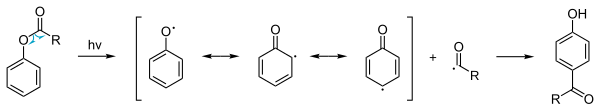
The Fries rearrangement proceeds through ionic intermediates. The reaction depends on the structure of the substrates and the reaction conditions.
The scheme depicts the formation of an ortho-acylated phenol from a substituted phenolic ester in the presence of aluminium trihalide catalyst. The photo Fries rearrangement mechanism proceeds through Radical intermediates.

The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenyl ester to a hydroxy aryl ketone by catalysis of Lewis acids.[1][2][3][4]
It involves migration of an acyl group of phenyl ester to benzene ring. The reaction is ortho and para selective and one of the two products can be favoured by changing reaction conditions, such as temperature and solvent.
Mechanism
Despite many efforts a definitive reaction mechanism for the Fries rearrangement is not available. Evidence for inter- and intramolecular mechanisms have been obtained by so-called cross-experiments with mixed reactants. Reaction progress is not dependent on solvent or substrate. A widely accepted mechanism involves a carbocation intermediate.In the first reaction step a Lewis acid for instance aluminium chloride AlCl
3 co-ordinates to the carbonyl oxygen atom of the acyl group. This oxygen atom is more electron rich than the phenolic oxygen atom and is the preferred Lewis base. This interaction polarizes the bond between the acyl residue and the phenolic oxygen atom and the aluminium chloride group rearranges to the phenolic oxygen atom. This generates a free acylium carbocation which reacts in a classical electrophilic aromatic substitution with the aromatic ring. The abstracted proton is released as hydrochloric acid where the chlorine is derived from aluminium chloride. The orientation of the substitution reaction is temperature dependent. A low reaction temperature favors para substitution and with high temperatures the ortho product prevails. Formation of the ortho product is also favoured in non-polar solvents; as the solvent polarity increases, the ratio of the para product also increases.[5]
Scope
Phenols react to esters but do not react to hydroxyarylketones with acylhalogen compounds under Friedel-Crafts acylation reaction conditions and therefore this reaction is of industrial importance for the synthesis of hydroxyarylketones which are important intermediates for several pharmaceutics such as paracetamol and salbutamol. As an alternative to aluminium chloride, other Lewis acids such as boron trifluoride and bismuth triflate or strong protic acids such as hydrogen fluoride and methanesulfonic acid can also be used. In order to avoid the use of these corrosive and environmentally unfriendly catalysts altogether research into alternative heterogeneous catalysts is actively pursued.Limits
In all instances only esters can be used with stable acyl components that can withstand the harsh conditions of the Fries rearrangement. If the aromatic or the acyl component is heavily substituted then the chemical yield will drop due to steric constraints. Deactivating meta-directing groups on the benzene group will also have an adverse effect as can be expected for a Friedel–Crafts acylation.Photo-Fries rearrangement
In addition to the ordinary thermal phenyl ester reaction a so-called photochemical Photo-Fries rearrangement exists[6] that involves a radical reaction mechanism. This reaction is also possible with deactivating substituents on the aromatic group. Because the yields are low this procedure is not used in commercial production. However, photo-Fries rearrangement may occur naturally, for example when a plastic bottle made of polyethylene terephthalate (PET) is exposed to the sun, particular to UV light at a wavelength of about 310 nm, if the plastic has been heated to 40 degrees Celsius or above (as might occur in a car with windows closed on a hot summer day). In this case, photolysis of the ester groups would lead to leaching of phthalate from the plastic.[7]Anionic Fries rearrangment
In addition to Lewis acid and photo-catalysed Fries rearrangements, there also exists an anionic Fries rearrangement. In this reaction, the aryl ester undergoes ortho-metallation with a strong base, which then rearranges in a nucleophilic attack mechanism.- Fries, K. ; Finck, G. (1908). "Über Homologe des Cumaranons und ihre Abkömmlinge". Chemische Berichte 41 (3): 4271–4284. doi:10.1002/cber.190804103146.
- Fries, K.; Pfaffendorf, W. (1910). "Über ein Kondensationsprodukt des Cumaranons und seine Umwandlung in Oxindirubin". Chemische Berichte 43 (1): 212–219. doi:10.1002/cber.19100430131.
- March, J. Advanced Organic Chemistry, 3rd Ed.; John Wiley & Sons: Chichester, 1985; S. 499ff.
- Blatt, A. H. Org. React. 1942, 1.
- Kürti, László; Czakó, Barbara (2005). Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms. Elsevier Academic Press. p. 181. ISBN 0123694833.
- Bellus, D. Advances in Photochemistry; John Wiley & Sons: Chichester, 1971; Vol. 8, 109–159.
- Norma Searle, "Environmental effects on polymeric materials," pp. 313–358, in Plastics and the Environment, edited by Anthony Andrade, Wiley, 2003.