
cyclopentanecarbaldehyde
| Cyclopentanecarbaldehyde | |
| Formula: | C6H10O |
| CAS#: | 872-53-7 |
| MW: シクロペンタンカルバルデヒド | 98.14 |
 Reference(s)Journal of the American Chemical Society, 96, p. 1623, 1974 DOI: 10.1021/ja00812a074 Synthesis, p. 395, 1988 Tetrahedron Letters, 11, p. 5275, 1970 | ||||||||||||||||||
Ciccioli, P., Brancaleoni, E., Cecinato, A., Sparapani, R., and Frattoni, M. 1993. Identification and determination of biogenic and anthropogenic volatile organic compounds in forest areas of northern and southern Europe and a remote site of the Himalaya region by high-resolution gas chromatography-mass spectrometry. J. Chromatogr. 643:55-69.Nomenclature of Aldehydes and Ketones
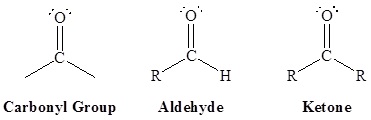
The most potent and varied odors are aldehydes. Ketones are widely used as industrial solvents. Aldehydes and ketones contain the carbonyl group. Aldehydes are considered the most important functional group. They are often called the formyl or methanoyl group. Aldehydes derive their name from the dehydration of alcohols. Aldehydes contain the carbonyl group bonded to at least one hydrogen atom. Ketones contain the carbonyl group bonded to two carbon atoms.
Introduction
Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group, C=O. The carbon atom of this group has two remaining bonds that may be occupied by hydrogen, alkyl or aryl substituents. If at least one of these substituents is hydrogen, the compound is analdehyde. If neither is hydrogen, the compound is a ketone.
 Naming Aldehydes
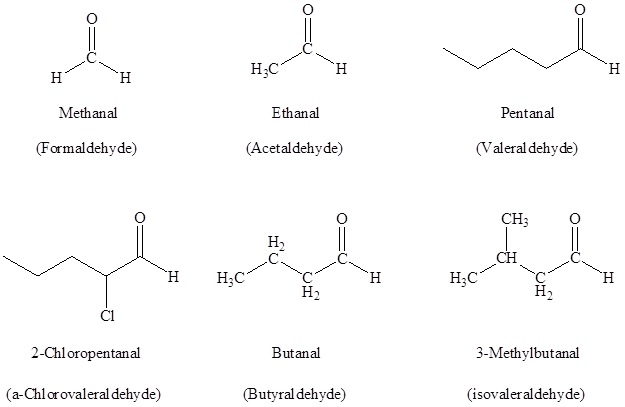
The IUPAC system of nomenclature assigns a characteristic suffix -al to aldehydes. For example, H2C=O is methanal, more commonly called formaldehyde. Since an aldehyde carbonyl group must always lie at the end of a carbon chain, it is always is given the #1 location position in numbering and it is not necessary to include it in the name. There are several simple carbonyl containing compounds which have common names which are retained by IUPAC.
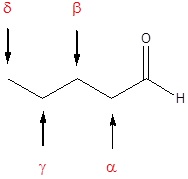
Also, there is a common method for naming aldehydes and ketones. For aldehydes common parent chain names, similar to those used for carboxylic acids, are used and the suffix –aldehyde is added to the end. In common names of aldehydes, carbon atoms near the carbonyl group are often designated by Greek letters. The atom adjacent to the carbonyl function is alpha, the next removed is beta and so on.
If the aldehyde moiety
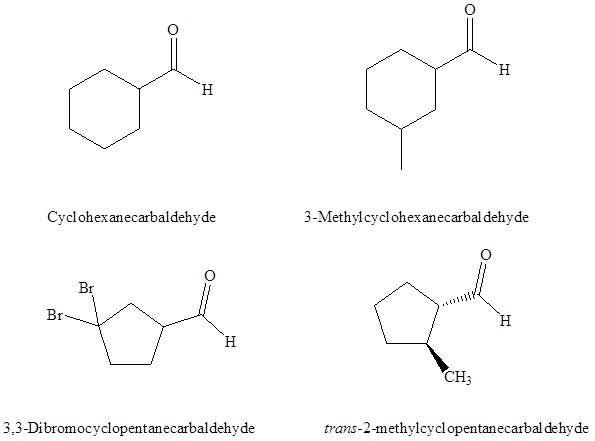
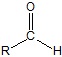 (-CHO) is attached to a ring the suffix –carbaldehyde is added to the name of the ring. The carbon attached to this moiety will get the #1 location number in naming the ring. (-CHO) is attached to a ring the suffix –carbaldehyde is added to the name of the ring. The carbon attached to this moiety will get the #1 location number in naming the ring.Summary of Aldehyde Nomenclature rules
Naming Ketones
The IUPAC system of nomenclature assigns a characteristic suffix of -one to ketones. A ketone carbonyl function may be located anywhere within a chain or ring, and its position is usually given by a location number. Chain numbering normally starts from the end nearest the carbonyl group. Very simple ketones, such as propanone and phenylethanone do not require a locator number, since there is only one possible site for a ketone carbonyl function
The common names for ketones are formed by naming both alkyl groups attached to the carbonyl then adding the suffix -ketone. The attached alkyl groups are arranged in the name alphabetically.
Summary of Ketone Nomenclature rules
Naming Aldehydes and Ketones in the Same Molecule
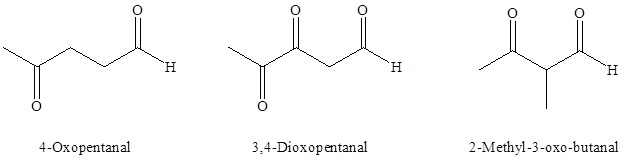
As with many molecules with two or more functional groups, one is given priority while the other is named as a substituent. Because aldehydes have a higher priority than ketones, molecules which contain both functional groups are named as aldehydes and the ketone is named as an "oxo" substituent. It is not necessary to give the aldehyde functional group a location number, however, it is usually necessary to give a location number to the ketone.
Naming Dialdehydes and Diketones
For dialdehydes the location numbers for both carbonyls are omitted because the aldehyde functional groups are expected to occupy the ends of the parent chain. The ending –dial is added to the end of the parent chain name.
For diketones both carbonyls require a location number. The ending -dione or -dial is added to the end of the parent chain.
Naming Cyclic Ketones and Diketones
In cyclic ketones the carbonyl group is assigned location position #1, and this number is not included in the name, unless more than one carbonyl group is present. The rest of the ring is numbered to give substituents the lowest possible location numbers. Remember the prefixcyclo is included before the parent chain name to indicate that it is in a ring. As with other ketones the –e ending is replaced with the –one to indicate the presence of a ketone.
With cycloalkanes which contain two ketones both carbonyls need to be given a location numbers. Also, an –e is not removed from the end but the suffix –dione is added.
Naming Carbonyls and Hydroxyls in the Same Molecule
When and aldehyde or ketone is present in a molecule which also contains an alcohol functional group the carbonyl is given nomenclature priority by the IUPAC system. This means that the carbonyl is given the lowest possible location number and the appropriate nomenclature suffix is included. In the case of alcohols the OH is named as a hydroxyl substituent. However, the l in hydroxyl is generally removed.
Naming Carbonyls and Alkenes in the Same Molecule
When and aldehyde or ketone is present in a molecule which also contains analkene functional group the carbonyl is given nomenclature priority by the IUPAC system. This means that the carbonyl is given the lowest possible location number and the appropriate nomenclature suffix is included.
When carbonyls are included with an alkene the following order is followed:
(Location number of the alkene)-(Prefix name for the longest carbon chain minus the -ane ending)-(an -en ending to indicate the presence of an alkene)-(the location number of the carbonyl if a ketone is present)-(either an –one or and -anal ending).
Remember that the carbonyl has priority so it should get the lowest possible location number. Also, remember that cis/tran or E/Z nomenclature for the alkene needs to be included if necessary.
Aldehydes and Ketones as Fragments
Additional Examples of Carbonyl Nomenclature
1) Please give the IUPAC name for each compound:
Answers for Question 1
2) Please give the structure corresponding to each name:
A) Butanal
B) 2-Hydroxycyclopentanone
C) 2,3-Pentanedione
D) 1,3-Cyclohexanedione
E) 3,4-Dihydoxy-2-butanone
F) (E)-2-Hepten-4-one
G) 3-Oxobutanal
H) cis-3-Bromocyclohexanecarboaldehyde
I) Butanedial
J) trans-2-methyl-3-Hexenal
Answers to question 2: References
|
 fragment, though the older naming, acyl, is still widely used.
fragment, though the older naming, acyl, is still widely used. fragment.
fragment.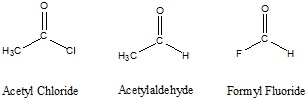
No comments:
Post a Comment