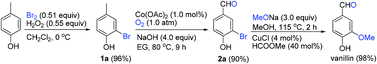Kinetic Resolution of Diols by Etherification
 Total synthesis of (+)-sacidumlignan D through
etherification-based kinetic resolution
Total synthesis of (+)-sacidumlignan D through
etherification-based kinetic resolutionRead more
Organic Synthesis International by Dr Anthony Melvin Crasto Ph.D, Worlddrugtracker, Million hits on google on all sites, One lakh connections worldwide. Pushing boundaries.Interaction site for Organic chemists worldwide, Mail me at amcrasto@gmail.com if you like me
 Total synthesis of (+)-sacidumlignan D through
etherification-based kinetic resolution
Total synthesis of (+)-sacidumlignan D through
etherification-based kinetic resolution