Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02443A, Paper
DOI: 10.1039/C5GC02443A, Paper
Ashish Anand, Jayashree Yenagi, J. Tonannavar, Manohar V. Kulkarni
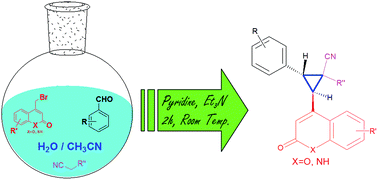
A one-pot three component reaction has been developed for the synthesis of substituted cyclopropanes employing 4-bromomethyl-2H-chromen-2-one/quinolin-2(1H)-ones, aromatic aldehydes and activated nitriles.
A one-pot three component reaction has been developed for the synthesis of substituted cyclopropanes employing 4-bromomethyl-2H-chromen-2-one/quinolin-2(1H)-ones, aromatic aldehydes and activated nitriles.
A one-pot three component reaction has been developed for the synthesis of substituted cyclopropanes employing 4-bromomethyl-2H-chromen-2-one/quinolin-2(1H)-ones, aromatic aldehydes and activated nitriles. The room temperature reaction in aqueous medium has been found to be diastereoselective and high yielding.
http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C5GC02443A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
2-(6-Methyl-2-oxo-2H-chromen-4-yl)-3-phenyl-cyclopropane-1,1-dicarbonitrile (4a)
4a, (R= -H, R’= 6-CH3, R”= -CN, X=O)
For a typical compound 4a, (R= -H, R’= 6-CH3, R”= -CN, X=O) IR spectrum showed band at 2246 cm-1 (cyanide) and 1720 cm-1 (lactone). Further, the formation of cyclopropane ring was confirmed by the 1H-NMR wherein the C5-H of coumarin ring resonated at 7.83 ppm as a doublet with 5JC5-H = 1.2 Hz showing a 5-bond coupling with the C4-CH of coumarin ring. C4- CH of coumarin ring appeared at 4.83 ppm as doublet of doublets with Jvic(CH) = 8.8 Hz, Cou-CH 5JC5-H = 1.2 Hz. CH attached to aryl group resonated at 4.61 ppm as a doublet with Jvic(CH) = 8.8 Hz. C6-CH3 protons appeared at 2.45 as a singlet. C3-H was observed as a singlet at 6.82 ppm. Other aromatic protons resonated between 7.68-7.41 ppm. In 13C-NMR, the carbon attached to two cyano group resonated at 15.08 ppm, the methyl carbon appeared at 20.55 ppm. Cyclopropane ring carbon attached to aryl group resonated at 32.81 ppm whereas the one attached to coumarin appeared at 36.78 ppm. Carbon of two cyano group resonated at 112.68 and 112.91 ppm. The lactone carbon of the coumarin ring appeared at 159.35 ppm. Aromatic carbons resonated in the range of 112-159 ppm. Formation of the product was further confirmed by EI-MS where the molecular ion peak was observed at 326 m/z
2-(6-Methyl-2-oxo-2H-chromen-4-yl)-3-phenyl-cyclopropane-1,1-dicarbonitrile (4a) White; Yield 85%;
m.p: 268-270°C;
IR (KBr) cm-1 1720 (C=O lactone), 2246 (CN);
1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 7.83(d, 1H, 5JC5-H = 1.2 Hz, C5-H), 7.68-7.41(m, 7H, ArH), 6.82(s, 1H, C3-H), 4.83(dd, 1H, Jvic(CH) = 8.8Hz, Cou-CH, 5JC5-H = 1.2 Hz), 4.61(d, 1H, Jvic(CH) = 8.8Hz, Ar-CH), 2.45(s, 3H, -CH3);
13C-NMR (100 MHz, DMSO-d6) δ (ppm): 8.57, 15.08, 20.55, 32.81, 36.78, 45.73, 112.68, 112.91, 115.95, 116.69, 117.64, 124.60, 128.62, 129.00,129.12, 130.90, 133.63, 134.23, 146.64, 151.12, 159.35;
MS m/z 326(100%);
Anal Calcd. for C21H14N2O2 (%), Calcd: C, 77.29; H, 4.32; N, 8.58; found: C, 77.26; H, 4.29; N, 8.55
Cyclopropanes in water: a diastereoselective synthesis of substituted 2H-chromen-2-one and quinolin-2(1H)-one linked cyclopropanes
Cyclopropanes in water: a diastereoselective synthesis of substituted 2H-chromen-2-one and quinolin-2(1H)-one linked cyclopropanes
*
Corresponding authors
a
Department of Studies in Chemistry, Karnatak University, Pavate Nagar, Dharwad 580003, India
E-mail: manohar274@gmail.com
E-mail: manohar274@gmail.com
b
Department of Studies in Physics, Karnatak University, Pavate Nagar, Dharwad 580003, India
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02443A http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C5GC02443A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract http://www.rsc.org/suppdata/c5/gc/c5gc02443a/c5gc02443a1.pdf ///////////////////////// c1(ccc4c(c1C2[C@@H](C2(C#N)C#N)c3ccccc3)\C=C/C(O4)=O)C



