| SYNTHESIS Highlight |
|
Synthesis
DOI: 10.1055/s-0035-1562579
DOI: 10.1055/s-0035-1562579
paper
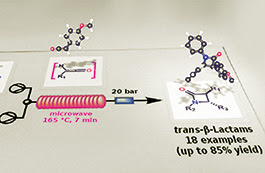
© Georg Thieme Verlag Stuttgart · New YorkCombination of Enabling Technologies to Improve and Describe the Stereoselectivity of Wolff–Staudinger Cascade Reaction
https://www.thieme-connect.de/products/ejournals/html/10.1055/s-0035-1562579?update=true
Abstract
A new, single-mode bench-top resonator was evaluated for the microwave-assisted flow generation of primary ketenes by thermal decomposition of α-diazoketones at high temperature. A number of amides and β-lactams were obtained by ketene generation in situ and reaction with amines and imines, respectively, in good to excellent yields. The preferential formation of trans-configured β-lactams was observed during the [2+2] Staudinger cycloaddition of a range of ketenes with different imines under controlled reaction conditions. Some insights into the mechanism of this reaction at high temperature are reported, and a new web-based molecular viewer, which takes advantage from Augmented Reality (AR) technology, is also described for a faster interpretation of computed data.


N-Benzyl-2-[4-(trifluoromethyl)phenyl]acetamide (4a)
Yield: 65%; white solid; mp 134–136 °C.
1H NMR (600 MHz, CDCl3): δ = 7.61 (d, J = 8.05 Hz, 2 H), 7.41 (d, J = 8.00 Hz, 2 H), 7.32 (d, J = 7.53 Hz, 2 H), 7.28–7.34 (m overlapping d at 7.32 ppm, 1 H), 7.22 (d, J = 7.13 Hz, 2 H), 5.89 (br. s, 1 H), 4.43 (d, J = 5.8 Hz, 2 H), 3.64 (s, 2 H).
13C NMR (150.0 MHz, CDCl3): δ = 169.7, 138.8 (br s), 137.8, 129.6, 129.59 (q, J C–F = 32.5 Hz), 128.7, 127.62, 127.59, 125.8 (q, J C–F = 3.8 Hz), 124.0 (q, J C–F = 272.0 Hz), 43.8, 43.3.
IR (neat): 3238, 3063, 1625, 1556, 1328, 1122, 1070, 753, 698 cm–1.
HRMS: m/z [M + H]+ calcd for C15H15F3NO: 294.1100; found: 294.1090.
/////////////////