Fluoxastrobin

Fluoxastrobin
Fluoxastrobin; Disarm; Fluoxastrobin [ISO]; UNII-XQ43WY091Y; HEC 480 SC;
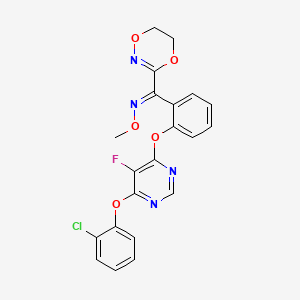
(E)-1-[2-[6-(2-chlorophenoxy)-5-fluoropyrimidin-4-yl]oxyphenyl]-1-(5,6-dihydro-1,4,2-dioxazin-3-yl)-N-methoxymethanimine
- (1E)-(2-((6-(2-chlorophenoxy)-5-fluoro-4-pyrimidinyl)oxy)phenyl)(5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyloxime
- 361377-29-9
- 193740-76-0
| Molecular Formula: | C21H16ClFN4O5 |
|---|---|
| Molecular Weight: | 458.826943 g/mol |
103-108 deg C
MacBean
C, ed; e-Pesticide Manual. 15th ed., ver. 5.1, Alton, UK; British Crop
Protection Council. Fluoxastrobin (361377-29-9) (2008-2010)
http://fluoridealert.org/wp-content/pesticides/fluoxastrobin.2004.article.pdf
http://fluoridealert.org/wp-content/pesticides/fluoxastrobin.2004.article.pdf
PAPER
http://pubs.rsc.org/en/content/articlehtml/2015/gc/c5gc00402k
PATENT
US-9193698-B2 / 2015-11-24
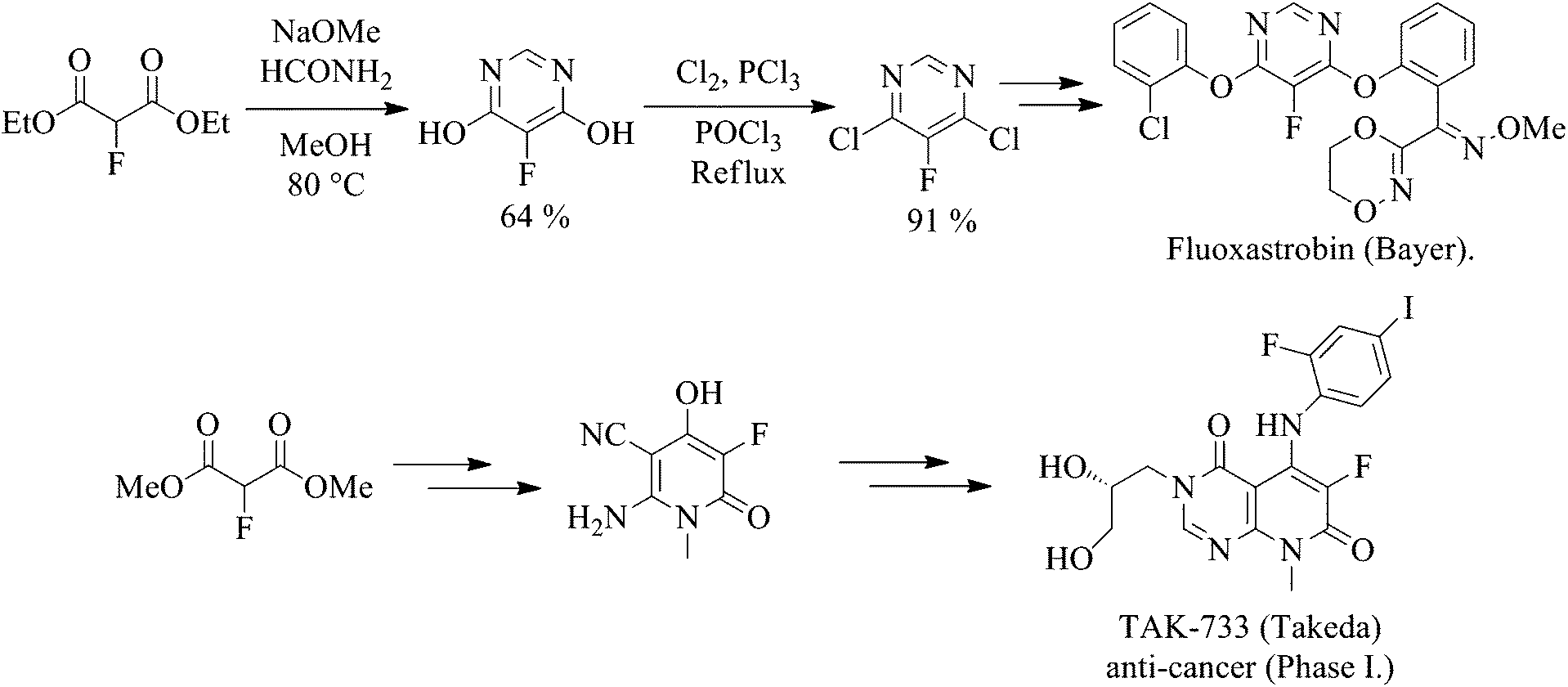
Process for preparing fluoxastrobin
(E)-(2-((6-(2-chlorophenoxy)-5-fluoropyrimidin-4-yl)oxy)phenyl)(5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyl oxime [Fluoxastrobin]
To a solution of (E)-(2-((6-chloro-5-fluoropyrimidin-4-yl)oxy)phenyl)(5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyl oxime (14)(100 g, 0.564 mol) in toluene was added 2-chlorophenol (54 g, 0.846 mol), K2CO3 (50
g, 0.733 mol), and DMF (50 mL) at ambient temperature. The reaction
mixture was stirred at 50-60° C. for 3-4 h. The progress of the reaction
was monitored by the HPLC analysis. Upon completion of the reaction,
aqueous NaOH (10%) (200 mL) was charged followed by water (300 mL). The
mixture was stirred and the toluene layer was separated. The toluene layer was washed with a solution of brine (600 mL). The final toluene layer was recovered completely to get the crude product. To the above crude product, methanol
was charged and heated to 60° C. until the clear solution is formed.
The solution was stirred at room temperature to get the pure product
precipitated. The pure fluoxastrobin product was filtered and washed with methanol. The product was further dried to obtain the pure fluoxastrobin product meeting the desired specifications. Yield—75-88%.
IR (cm−1,
KBr) 3072.99w, 2981.58w, 2936.76s, 2819.79w, 2502.01w, 1601.14s,
1572.37s, 1447.88s, 1305.43m, 1268.11m, 1217.15m, 1191.21m, 1092.60m,
1049.05m, 1001.26w, 910.25w, 762.81w.
1H NMR (CDCl3,
400 MHz) δ 3.846 (s, 3H), 4.170-4.160 (t, J=4 Hz, 2H), 4.464-4.484 (t,
J=4 Hz, 2H), 7.261-7.295 (m, 2H), 7.322-7.409 (2, 4H), 8.069 (s, 1H).
13C NMR (CDCl3,
400 MHz) δ 63.103, 64.153, 64.550, 122.659, 123.259, 123.823, 125.712,
127.150, 127.397, 128.094, 130.511, 130.679, 130.776, 131.473, 134.138,
146.004, 148.166, 148.943, 150.354, 150.478, 151.819, 157.395, 157.466,
157.783, 157.854.
MS (EI) m/z 459.1 (M+1); MS2 (EI) m/z 427.1, 383.0,
366.9, 342.1, 306.2, 246.0, 231.1, 188.0.
HPLC (Area %): 99.40%. M.P.
108-112° C.
Isomerisation of (Z)-Fluoxastrobin to (E)-Fluoxastrobin using methane sulphonic acid. To a stirred solution of (Z)-Fluoxastrobin (0.3 g; 0.65 mmole) in acetonitrile (3 ml) was dropwise added methane sulphonic acid
(0.04 ml, 0.65 mmole) at an ambient temperature. The reaction mixture
was stirred for 2-3 hr at the same temperature. The progress of reaction
was monitored by thin layer chromatography (TLC). Dichloromethane
(5 ml) and DM water (5 ml) was added to reaction mass at an ambient
temperature. After vigorous stirring, the layers were separated. The
aqueous layer was back extracted with dichloromethane (5 ml) and the combined dichloromethane layer was washed with 10% aqueous sodium bicarbonate solution (20 ml) followed by washing with 10% brine solution (20 ml). Dichloromethane
was distilled off at reduced pressure at 35-45° C. to obtain
(E)-Fluoxastrobin as crude product (0.25 g, 83% of theoretical yield).
Crude fluoxastrobin on purification in ethanol affords pure (E)-Fluoxastrobin. Isolated product HPLC purity (% area): (Z)-fluoxastrobin: 1.02% and (E)-fluoxastrobin: 95.92%.
Isomerisation of (Z)-Fluoxastrobin to (E)-Fluoxastrobin using phosphoric acid. To a stirred solution of (Z)-Fluoxastrobin (0.25 g; 0.54 mmole) in acetonitrile (4 ml) was dropwise added phosphoric acid
(0.03 g, 0.54 mmole) at an ambient temperature. The reaction mixture
was stirred for 2-3 hr at the same temperature. Progress of reaction was
monitored by thin layer chromatography/HPLC. Dichloromethane
(5 ml) and DM water (5 ml) was added to reaction mass at an ambient
temperature. After vigorous stirring, layers were separated. The aqueous
layer was back extracted with dichloromethane (5 ml). The combined dichloromethane layers were washed with 10% aq. Sodium bicarbonate solution (20 ml) followed by washing with 10% brine solution (20 ml). Dichloromethane
was distilled off at reduced pressure at 40-45° C. to obtained
(E)-Fluoxastrobin (0.22 g, 88% of Theoretical yield). Reaction
monitoring by HPLC (% area): (Z)-Fluoxastrobin: 6.79% and
(E)-Fluoxastrobin: 88.84%. Isolated product HPLC purity (% area):
(Z)-Fluoxastrobin: 6.94% and (E)-Fluoxastrobin: 84.43%.
IR (cm−1,
KBr) 3066.28w, 2981.58w, 2939.36s, 2825.71w, 2500.61w, 1602.36s,
1572.76s, 1441.05s, 1297.05m, 1218.17m, 1116.52s, 1046.15m 1000.86w,
904.73s, 764.71w. 1H NMR (CDCl3, 400 MHz) δ 3.983
(s, 3H), 4.163-4.218 (t, 2H), 4.432-4.440 (t, J=3.2 Hz, 2H), 7.217-7.352
(m, 4H), 7.371-7.390 (m, 2H), 7.483-7.516 (m, 2H), 7.702-7.722 (d, J=8
Hz, 1H), 8.016 (s, 1H). MS (EI) m/z 459.1 (M+1); MS2 (EI) m/z 427.0,
382.9, 366.7, 340.0, 305.8, 246.1, 188.0. HPLC (Area %): 99.11%. M.P.
150-152° C.
Title: Fluoxastrobin
CAS Registry Number: 361377-29-9
CAS Name: (1E)-[2-[[6-(2-Chlorophenoxy)-5-fluoro-4-pyrimidinyl]oxy]phenyl](5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyloxime
Manufacturers' Codes: HEC-5725
Trademarks: Fandango (Bayer CropSci.)
Molecular Formula: C21H16ClFN4O5
Molecular Weight: 458.83
Percent Composition: C 54.97%, H 3.51%, Cl 7.73%, F 4.14%, N 12.21%, O 17.43%
Literature References:
Leaf-systemic broad-spectrum fungicide for use in cereal and food
crops; member of methoxyimiodihydro-dioxazines. Prepn (stereochem.
unspecified): U. Heinemann et al., DE 19602095; eidem, US 6103717 (1997, 2000 both to Bayer). Comprehensive description: S. Dutzmann et al., BCPC Conf. - Pests Dis. 2002, 365. Field trial in winter wheat seeds: I. Haeuser-Hahn et al., BCPC Int. Cong. - Crop Sci. Tech. 2003, 801. Series of articles on chemistry, biology, determn, and environmental fate: Pflanzenschutz-Nachr. Bayer (Engl. Ed.) 57, 299-449 (2004). Ecotoxicology: P. Breuer, ibid. 319.
Properties: White crystals with slight characteristic odor, mp 103-108°. bp 497° (est.). d420 1.422. Log P (octanol/water): 2.86 (20°). Vapor pressure at 20° (extrapolated): 6 ´ 10-10 Pa. Soly at 20° (g/l): n-heptane
0.04; 2-propanol 6.7; xylene 38.1; dichloromethane >250; in water
(mg/l): 2.56 (unbuffered); 2.43 (pH 4); 2.29 (pH 7); 2.27 (pH 9). LD50 in rats, bobwhite quail (mg/kg): >2500, >2000 orally; LC50 (96 hr) rainbow trout, bluegill sunfish, carp (mg/l): 0.44, 0.97, 0.57 (Breuer).
Melting point: mp 103-108°
Boiling point: bp 497° (est.)
Log P: Log P (octanol/water): 2.86 (20°)
Density: d420 1.422
Toxicity data: LD50 in rats, bobwhite quail (mg/kg): >2500, >2000 orally; LC50 (96 hr) rainbow trout, bluegill sunfish, carp (mg/l): 0.44, 0.97, 0.57 (Breuer)
Use: Agricultural fungicide.



