 .
.Hydrogenative cyclization of levulinic acid into [gamma]-valerolactone by photocatalytic intermolecular hydrogen transfer
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02971F, Communication
DOI: 10.1039/C5GC02971F, Communication
Hongxia Zhang, Min Zhao, Tianjian Zhao, Li Li, Zhenping Zhu
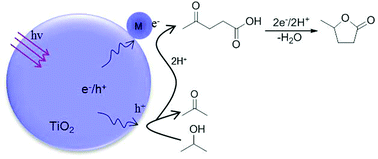
A hydrogenation-dehydrogenation coupling process efficiently realized an intermolecular hydrogen transfer from isopropanol to LA under photocatalytic conditions over gold-loaded TiO2 catalysts.
A hydrogenation-dehydrogenation coupling process efficiently realized an intermolecular hydrogen transfer from isopropanol to LA under photocatalytic conditions over gold-loaded TiO2 catalysts.
click
http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C5GC02971F?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
The hydrogenative cyclization of levulinic acid (LA) into γ-valerolactone (GVL) is an attractive route toward the use of renewable bio-sources but it normally suffers from the consumption of H2. In this study, we report that an intermolecular hydrogen transfer from isopropanol to LA can be realized efficiently under photocatalytic conditions over gold-loaded TiO2 catalysts. In this manner, isopropanol is dehydrogenated as acetone and pinacol with the total selectivity of >99%, whereas LA is hydrogenated and cyclized as GVL with the selectivity of up to 85%. In this reaction process, the production of GVL is mediated with hydrogenated dehydration of LA into an acetyl propionyl radical, which is further hydrogenated and cyclized as GVL. This hydrogenation–dehydrogenation coupling process provides an atom-economical green way for the conversion of LA into GVL.
Hydrogenative cyclization of levulinic acid into γ-valerolactone by photocatalytic intermolecular hydrogen transfer
*
Corresponding authors
a
Institute of Application Chemistry, Shanxi University, Taiyuan 030006, China
E-mail: hxzhang@sxu.edu.cn
E-mail: hxzhang@sxu.edu.cn
b
State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China
E-mail: zpzhu@sxicc.ac.cn
E-mail: zpzhu@sxicc.ac.cn
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02971F
///////