Recent advances in trifluoromethylation of organic compounds using Umemoto's reagents
Org. Biomol. Chem., 2014, 12,6580-6589
DOI: 10.1039/C4OB00671B, Review Article
DOI: 10.1039/C4OB00671B, Review Article
Cai Zhang
This review highlights recent developments in the direct introduction of a trifluoromethyl group into organic compounds with Umemoto's reagents
DOI: 10.1039/C4OB00671B
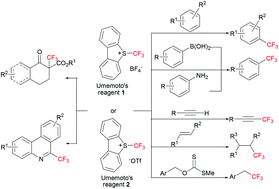
The incorporation of fluorine-containing moieties into organic compounds is of great importance in pharmaceutical, agricultural, and materials science. Within these organofluorides, the trifluoromethyl group is one of the most important motifs. In recent years, the trifluoromethyl group has attracted more and more attention, and many trifluoromethylated compounds have been found to possess special activities. However, until now, only a few methods have been developed to achieve this efficiently using Umemoto's reagents. This review highlights recent developments in the direct introduction of a trifluoromethyl group into organic compounds with Umemoto's reagents. Seven approaches to the trifluoromethylation of organic compounds are summarized: (i) trifluoromethylation of arenes, (ii) trifluoromethylation of alkenes, (iii) trifluoromethylation of terminal alkynes, (iv) deoxygenative trifluoromethylation of benzylic xanthates, (v) trifluoromethylation of ketoesters, (vi) trifluoromethylation of aryl boronic acids and aromatic amines (synthesis of ArCF3) and (vii) trifluoromethylation of biphenyl isocyanide derivatives.
This review highlights recent developments in the direct introduction of a trifluoromethyl group into organic compounds with Umemoto's reagents
a
Department of Resources and Environment, Chongqing Vocational Institute of Safety Technology, 583 Anqing Road, Wanzhou District, Chongqing, P. R. China
E-mail: stezh64@163.com;
Fax: (+86)23-58567750 ;
Tel: (+86)23-58567778
E-mail: stezh64@163.com;
Fax: (+86)23-58567750 ;
Tel: (+86)23-58567778
Org. Biomol. Chem., 2014,12, 6580-6589
DOI: 10.1039/C4OB00671B
The incorporation of fluorine-containing moieties into organic compounds is of great importance in pharmaceutical, agricultural, and materials science. Within these organofluorides, the trifluoromethyl group is one of the most important motifs. In recent years, the trifluoromethyl group has attracted more and more attention, and many trifluoromethylated compounds have been found to possess special activities. However, until now, only a few methods have been developed to achieve this efficiently using Umemoto's reagents. This review highlights recent developments in the direct introduction of a trifluoromethyl group into organic compounds with Umemoto's reagents. Seven approaches to the trifluoromethylation of organic compounds are summarized: (i) trifluoromethylation of arenes, (ii) trifluoromethylation of alkenes, (iii) trifluoromethylation of terminal alkynes, (iv) deoxygenative trifluoromethylation of benzylic xanthates, (v) trifluoromethylation of ketoesters, (vi) trifluoromethylation of aryl boronic acids and aromatic amines (synthesis of ArCF3) and (vii) trifluoromethylation of biphenyl isocyanide derivatives.
No comments:
Post a Comment