Green Chem., 2016, 18,2642-2646
DOI: 10.1039/C6GC00497K, Communication
Xiaofeng Zhang, Sanjun Zhi, Wei Wang, Shuai Liu, Jerry P. Jasinski, Wei Zhang
A pot-economical synthesis involving two [3 + 2] cycloadditions for diastereoselective synthesis of novel triazolobenzodiazepine-containing polycyclic compounds.
The content of this RSS Feed (c) The Royal Society of Ch
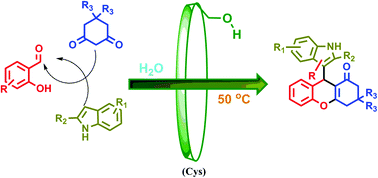
A pot-economical and diastereoselective synthesis involving catalyst-free click reaction for fused-triazolobenzodiazepines
a
Centre for Green Chemistry and Department of Chemistry, University of Massachusetts Boston, 100 Morrissey Boulevard, Boston, USA
E-mail: wei2.zhang@umb.edu
b
Jiangsu Key Laboratory for the Chemistry of Low-Dimensional Materials, Huaiyin Normal University, Huaian, PR China
c
School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an, PR China
d
Department of Chemistry, Keene State College, Keene, USA
Green Chem., 2016,18, 2642-2646
DOI: 10.1039/C6GC00497K
A pot-economical synthesis involving sequential [3 + 2] cycloadditions of an azomethine ylide and an azide–alkyne (click reaction) has been developed for diastereoselective synthesis of novel triazolobenzodiazepine-containing polycyclic compounds. A new example of catalyst-free click chemistry of non-strained alkynes is also disclosed.
DISCLAIMER
I , Dr A.M.Crasto is writing this blog to share the knowledge/views, after reading Scientific Journals/Articles/News Articles/Wikipedia. My views/comments are based on the results /conclusions by the authors(researchers). I do mention either the link or reference of the article(s) in my blog and hope those interested can read for details. I am briefly summarising the remarks or conclusions of the authors (researchers). If one believe that their intellectual property right /copyright is infringed by any content on this blog, please contact or leave message at below email address amcrasto@gmail.com. It will be removed ASAP
////