Marcelle S. Ferreira; José D. Figueroa-Villar*
Quim. Nova, Vol. 38, No. 2, 233-236, 2015
Artigo http://dx.doi.org/10.5935/0100-4042.20140308
*e-mail: jdfv2009@gmail.com
MÉTODO ALTERNATIVO PARA A SÍNTESE E MECANISMO DE 2-(1,3-DITIANO-2-ILIDENO)-ACETONITRILA
Marcelle S. Ferreira e José D. Figueroa-Villar* Departamento de Química, Instituto Militar de Engenharia, Praça General Tiburcio 80, 22290-270
Rio de Janeiro – RJ, Brasil
Recebido em 18/08/2014; aceito em 15/10/2014; publicado na web em 12/12/2014
ALTERNATIVE METHOD FOR SYNTHESIS AND MECHANISM OF 2-(1,3-DITHIAN-2-YLIDENE)-ACETONITRILE. We report an alternative method for the synthesis of 2-(1,3-dithian-2-ylidene)-acetonitrile using 3-(4-chlorophenyl)-3-oxopropanenitrile and carbon disulfide as starting materials. The methanolysis of the intermediate 3-(4-chlorophenyl)-2-(1,3-dithian-2-ylidene)-3- oxopropanenitrile occurs via three possible intermediates, leading to the formation of the product at a 75% overall yield. Molecular modeling simulation of the reaction pathway using B3LYP 6-311G++(2df,2p) justified the proposed reaction mechanism. Keywords: 2-(1,3-dithian-2-ylidene)-acetonitrile; reaction mechanism; methanolysis; molecular modeling.
3-(4-clorofenil)-2-(1,3-ditiano-2-ilideno)-3-oxopropanonitrila (3): Cristal amarelo. Rendimento: 95%, 2,80 g, pf 158-160 °C, lit.21 159-160 °C;
IV (KBr, cm-1): 2198 (CN), 1612 (C=O), 1585, 1560 (aromático), 678 cm -1 (C-S);
1H RMN (300 MHz, CDCl3) δ 2,38 (m, J 6,9, 2H, CH2); 3,01 (t, J 6,6, 2H, SCH2); 3,17 (t, J 7,2 , 2H, SCH2); 7,43 (d, J 8,5, 2H); 7,83 (d, J 8,5, 2H);
13C RMN (75 MHz, CDCl3) δ 23,9 (CH2), 30,4 (SCH2), 104,2 (CCO), 117,5 (CN), 128,9, 130,5, 135,6, 139,2 (aromático), 185,2 (C=CS), 185,4 (CO).
21.......Rudorf, W. D.; Augustin, M.; Phosphorus Sulfur Relat. Elem. 1981, 9, 329.
...........................................
Síntese da 2-(1,3-ditiano-2-ilideno)-acetonitrila (1) Em um balão de fundo redondo de 100 mL foram adicionados 0,400 g (1,4 mmol) de 3-(4-clorofenil)-2-(1,3-ditiano-2-ilideno)-3- -oxopropanonitrila (2) dissolvidos em 15 mL de THF seco, 0,140 g (20 mmol) de sódio e 15 mL de metanol seco sob atmosfera de nitrogênio. A mistura reacional foi mantida sob agitação à 25 °C por 48 h. Em seguida, a mistura reacional foi dissolvida em 30 mL de água destilada e extraída com acetato de etila (3 x 20 mL). A fase orgânica foi seca em sulfato de sódio anidro, filtrada e concentrada a vácuo para se obter o produto bruto, que foi purificado por cromatografia em coluna (silica gel e hexano:acetato de etila 7:3).
2-(1,3-ditiano-2-ilideno)-acetonitrila (1): Cristal branco. Rendimento: 75%, 165 mg, pf. 60-63 °C, lit1 60-62 °C;
1 H RMN (300 MHz, CDCl3) δ 2,23 (m, J 6,8, 2H, CH2); 3,01 (t, J 7,5, 2H, SCH2); 3,06 (t, J 6,9, 2H, SCH2), 5,39 (s, 1H, CH);
13C RMN (75 MHz, CDCl3) δ 22,9 (CH2), 28,7 (SCH2), 28,8 (SCH2), 90,4 (CHCN), 116,3 (CN), 163,8 (C=CS).
1.........Yin, Y.; Zangh, Q.; Liu, Q.; Liu, Y.; Sun, S.; Synth. Commun. 2007, 37, 703.
CAS 113998-04-2
- C6 H7 N S2
- Acetonitrile, 2-(1,3-dithian-2-ylidene)-
- 157.26
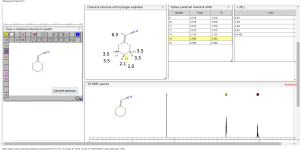
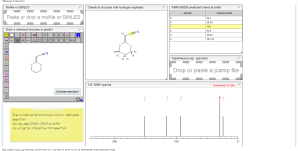
1H NMR predict
2-(1,3-dithian-2-ylidene)-acetonitrile
ACTUAL 1H NMR VALUES
1 H RMN (300 MHz, CDCl3)
δ 2,23 (m, J 6,8, 2H, CH2);
3,01 (t, J 7,5, 2H, SCH2);
3,06 (t, J 6,9, 2H, SCH2),
5,39 (s, 1H, CH);
..........................
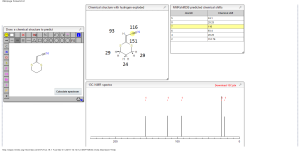
13C NMR PREDICT
ACTUAL 13C NMR VALUE
13C RMN (75 MHz, CDCl3)
δ 22,9 (CH2),
28,7 (SCH2),
28,8 (SCH2),
90,4 (CHCN),
116,3 (CN),
163,8 (C=CS)
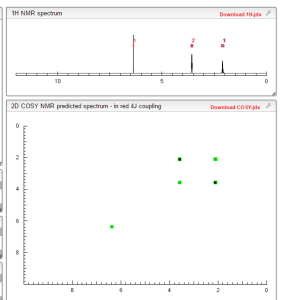
COSY NMR PREDICT
SYNTHESIS
2-(1,3-ditiano-2-ilideno)-acetonitrila (1): Cristal branco. Rendimento: 75%, 165 mg, pf. 60-63 °C, lit1 60-62 °C;
1 H RMN (300 MHz, CDCl3) δ 2,23 (m, J 6,8, 2H, CH2); 3,01 (t, J 7,5, 2H, SCH2); 3,06 (t, J 6,9, 2H, SCH2), 5,39 (s, 1H, CH);
13C RMN (75 MHz, CDCl3) δ 22,9 (CH2), 28,7 (SCH2), 28,8 (SCH2), 90,4 (CHCN), 116,3 (CN), 163,8 (C=CS).
WILL BE UPDATED WATCH OUT.....................
Departamento de Química, Instituto Militar de Engenharia, Praça General Tiburcio
Instituto Militar de Engenharia, Rio de Janeiro. BELOW
Entrada do antigo Instituto de Química da UFRGS, um prédio histórico
Equipe - Os módulos foram fabricados na Unisanta sob a supervisão do professor Luiz Renato Lia, coordenador do Curso de Engenharia Química, ...
Instituto de Florestas da Universidade Federal Rural do Rio de Janeiro
Praça General Tibúrcio
Praça General Tibúrcio com o Morro da Urca ao fundo
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.

 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE

 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE








 Building in which the College of Medicine operated until 1973, ...
...............
Building in which the College of Medicine operated until 1973, ...
...............
 A CHEAP AND EFFICIENT PHOTOCHEMICAL REACTOR FOR CHEMICAL EXPERIMENTS. In this work, we present an efficient and inexpensive device for undergraduate chemistry classes aimed at teaching and learning the photolytic synthesis concepts. A photochemical reactor was tested for the synthesis of the organometallic compound enneacarbonyldiiron from iron pentacarbonyl in acetic acid, and its formation evidenced by FTIR analysis. Although similar devices have been described in other studies, none of these offered the simplicity, low cost, class-compatible reaction times and good yields afforded by the procedure reported herein.
Keywords: photochemistry; reactor; enneacarbonyldiiron.
A CHEAP AND EFFICIENT PHOTOCHEMICAL REACTOR FOR CHEMICAL EXPERIMENTS. In this work, we present an efficient and inexpensive device for undergraduate chemistry classes aimed at teaching and learning the photolytic synthesis concepts. A photochemical reactor was tested for the synthesis of the organometallic compound enneacarbonyldiiron from iron pentacarbonyl in acetic acid, and its formation evidenced by FTIR analysis. Although similar devices have been described in other studies, none of these offered the simplicity, low cost, class-compatible reaction times and good yields afforded by the procedure reported herein.
Keywords: photochemistry; reactor; enneacarbonyldiiron.







 Campus Palhoça-Bilingue. Atualizado por Rafael Batista. O Instituto Federal de Educação, Ciência e Tecnologia de Santa Catarina ...
Campus Palhoça-Bilingue. Atualizado por Rafael Batista. O Instituto Federal de Educação, Ciência e Tecnologia de Santa Catarina ...

 Aberto o concurso do Instituto Federal de Educação, Ciência e Tecnologia de Santa Catarina (IFSC). A seleção visa prover um total de 145 vagas.
Aberto o concurso do Instituto Federal de Educação, Ciência e Tecnologia de Santa Catarina (IFSC). A seleção visa prover um total de 145 vagas.
 Instituto Federal de Educação, Ciência e Tecnologia - Campus Rio do Sul - Cantagalo
Instituto Federal de Educação, Ciência e Tecnologia - Campus Rio do Sul - Cantagalo