

Homochiral tricarbonyl chromium arenes in the stereoselective synthesis of heterocyclic compounds
Clara Baldoli, Paola Del Buttero and Stefano Maiorana
Dipartimento di Chimica Organica e Industriale dell'Universita' e Centro CNR, Università degli Studi di Milano, Via C. Golgi,19 -20133, Milano, Italy

Stefano Maiorana

Intermolecular [2+2] cycloaddition
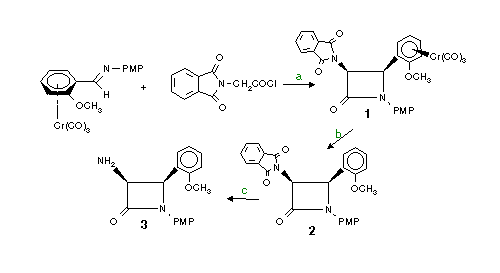
Synthesis of homochiral 3-amino azetidin-2-one
- Background
- Resolution of ortho -substituted benzaldehydes
- Reaction conditions
- Products
- Hypothesis for stereoselection

Background
Arene chromium tricarbonyl complexes are widely used in organic synthesis because they are synthetically equivalent to the free arene, yet possess different chemical properties.
Besides electronic effects, (due to the electron withdrawing character of the tricarbonyl chromium unit), the metal carbonyl moiety imparts a third dimension to the molecule: ortho and meta unsymmetrically disubstituted chromium tricarbonyl complexes are chiral and in many cases they can be resolved into the corresponding enantiomers.
Chiral tricarbonyl (ortho-substituted benzaldehyde)chromium complexes are the most widely applied examples in asymmetric synthesis.
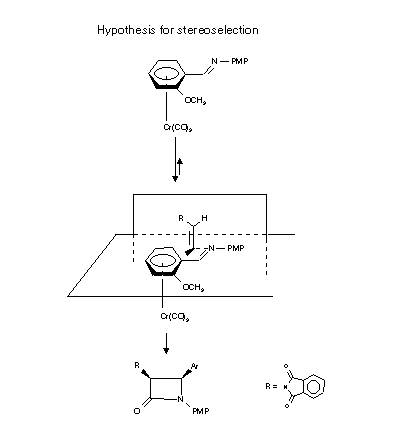
The accepted model for stereoselection is based on the fact that the metal spatially occupies one face of the ring and attack of any reagent at the benzylic position is constrained to approach only from the sideanti to the metal; in addition the carbonyl group lies preferentially in the anti conformation with o respect to the ortho substituent.
These two factors afford a high degree of stereoselectivity.
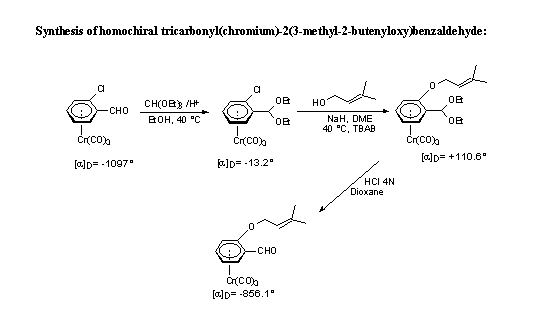
Resolution of ortho -substituted benzaldehydes
There have been several reported routes to optically active ortho-substituted tricarbonylchromium benzaldehyde complexes utilizing chemical or enzymatic resolutions.
One the most used methods involves a chromatographic separation and subsequent hydrolysis of diastereoisomeric derivatives obtained from (S)-5-(1-phenethyl)semioxamazide or from L-valinol.
- Lindsay, A.B.; Davies, S.G.; Craig, L.G. Tetrahedron: Asymmetry 1991, 2, 139.
- Solladie'-Cavallo, A,; Solladie', G.; Tsamo, E. J. Org. Chem. 1979, 44, 4189.
- Maiorana, S.; Jaouen, G., Baldoli, C.; Del Buttero, P., Top, S. J. Organomet. Chem. 1991, 416, 125.
- Yamazaki, Y.; Hosono, K. Tetrahedron Lett. 1990, 31, 3895.
- Nakamura, K; Ishihara, K.; Ohno, A.; Uemura, M.; Nushimura, H.; Hayashi, Y. Tetrahedron Lett. 1990, 31, 3603.
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
Reaction conditions
(a) dry Methylene chloride, 0 �C , TEA (reactant ratio: imine/TEA/N-phthaloylglycinyl chloride: 1/6.5/6)
(b) methylene chloride, room temp., sun light
(c) Hydrazine, MeOH, 37 �C, 3 h (reactant ratio: b-lactam/hydrazine: 1/4.5)
Products
1-N-p-methoxyphenyl-3-phthalymido-4(2-methoxyphenyl Cr(CO)3) azetidin-2-one
yield = 83% d.e.> 98% [a]D= -238 (c 0.1 CHCl3)
2-N-p-methoxyphenyl-3-phthalymido-4(2-methoxyphenyl ) azetidin-2-one
yield = 86% e.e.> 98% [a]D= -31 (c 1 CHCl3)
3-N-p-methoxyphenyl-3-amino-4(2-methoxyphenyl) azetidin-2-one
yield = 67% [a]D= -80 (c 0.1 CHCl3)
Intramolecular hetero-Diels-Alder reaction
Synthesis of homochiral cis-fused dihydropyran
Background
Arene chromium tricarbonyl complexes are widely used in organic synthesis because they are synthetically equivalent to the free arene, yet possess different chemical properties.
Besides electronic effects, (due to the electron withdrawing character of the tricarbonyl chromium unit), the metal carbonyl moiety imparts a third dimension to the molecule: ortho and meta unsymmetrically disubstituted chromium tricarbonyl complexes are chiral and in many cases they can be resolved into the corresponding enantiomers.
Chiral tricarbonyl (ortho-substituted benzaldehyde)chromium complexes are the most widely applied examples in asymmetric synthesis.
The accepted model for stereoselection is based on the fact that the metal spatially occupies one face of the ring and attack of any reagent at the benzylic position is constrained to approach only from the sideanti to the metal; in addition the carbonyl group lies preferentially in the anti conformation with o respect to the ortho substituent.
These two factors afford a high degree of stereoselectivity.
Resolution of ortho -substituted benzaldehydes
There have been several reported routes to optically active ortho-substituted tricarbonylchromium benzaldehyde complexes utilizing chemical or enzymatic resolutions.
One the most used methods involves a chromatographic separation and subsequent hydrolysis of diastereoisomeric derivatives obtained from (S)-5-(1-phenethyl)semioxamazide or from L-valinol.
- Lindsay, A.B.; Davies, S.G.; Craig, L.G. Tetrahedron: Asymmetry 1991, 2, 139.
- Solladie'-Cavallo, A,; Solladie', G.; Tsamo, E. J. Org. Chem. 1979, 44, 4189.
- Maiorana, S.; Jaouen, G., Baldoli, C.; Del Buttero, P., Top, S. J. Organomet. Chem. 1991, 416, 125.
- Yamazaki, Y.; Hosono, K. Tetrahedron Lett. 1990, 31, 3895.
- Nakamura, K; Ishihara, K.; Ohno, A.; Uemura, M.; Nushimura, H.; Hayashi, Y. Tetrahedron Lett. 1990, 31, 3603.
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
Reaction conditions
(a) dry CH2Cl2, room temp., 24 h (reactant ratio: aldehyde/Meldrum's acid/EDDA: 1/1.1/0.4)
(b) CH2Cl2, room temp., sunlight
Products
Cr(CO)3complexed cis-fused dihydropyran 1
yield 60% d.e.>98% [a]D= -106 (c 0.17 CHCl3)
cis-fused dihydropyran 2
yield 95% e.e.>98% [a]D= +87.9 (c 0.5 CHCl3)
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO







