
With
the plethora of new and efficient C–C bond-forming reactions available
to the organic chemists growing on a monthly basis, one area that
suffers is the substrate scope for previously reported examples. In this
case Kim and Reike ( Tetrahedron Lett. 2011, 52, 1523−1526)
reinvestigate work originally reported by Rovis. Initially Kim performs
a small catalyst screen using various commercially available catalysts,
resulting in Ni(acac)2 being chosen for the remaining
coupling reactions due to the rate of reaction and the isolated yield it
facilitated. With a large selection of organozinc reagents via direct
insertion developed by Reike, they then apply the developed conditions
to 26 examples, all of which gave isolated product in good to excellent
yields on gram scale.
Preparation of aryl ketones via Ni-catalyzed Negishi-coupling reactions with acid chlorides
- a Department of Chemistry, Dankook University, 29 Anseo, Cheonan 330-714, Republic of Korea
- b Rieke Metals, Inc., 1001 Kingbird Rd. Lincoln, NE 68521, USA
- http://www.sciencedirect.com/science/article/pii/S0040403911001845
Abstract
A
Ni-catalyst-catalyzed cross-coupling reaction of organozinc reagents
with acid chlorides has been successfully developed. Mild reaction
conditions were required to complete the coupling reactions affording
the corresponding aryl ketones in good to excellent yields.
Graphical abstract


A representative procedure of coupling reaction; In a 25 mL round-bottomed flask, Ni(acac)2, (0.06 g, 2 mol%) and 10 mL (5 mmol) of 0.5 M solution of 2- (ehtoxycarbonyl)phenylzinc bromide in THF was added into the flask at room temperature. Next, 6-chloronicotinoyl chloride (0.70 g, 4 mmol) dissolved in 5.0 mL of THF was added. The resulting mixture was refluxed overnight, then cooled down to room temperature. Quenched with saturated NH4Cl solution, then extracted with ethyl acetate (30 mL 3). Combined organics were washed with saturated Na2S2O3 solution and brine. Dried over anhydrous MgSO4. A flash column chromatography (50% EtOAc/50% Heptane) gave 0.78 g of 3g as yellow solid in 68% isolated.
Mp = 48–51 C. 1
H NMR (CDCl3, 500 MHz): d 8.59 (s, 1H), 8.11 (d, 2H, J = 10 Hz), 7.69 (t, 1H, J = 5 Hz), 7.62 (t, 1H, J = 5 Hz), 7.43 (d, 1H, J = 5 Hz), 7.38 (d, 1H, J = 10 Hz), 4.17 (q, 2H, J = 5 10 Hz), 1.19 (t, 3H, J = 10 Hz);
13C NMR (CDCl3, 125 MHz): d 194.8, 165.6, 155.6, 151.2, 140.6, 138.8, 133.0, 131.9, 130.6, 130.4, 129.2, 127.5, 124.6, 61.9, 14.0.
1H AND 13C NMR PREDICT
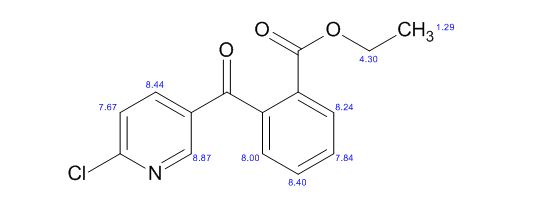
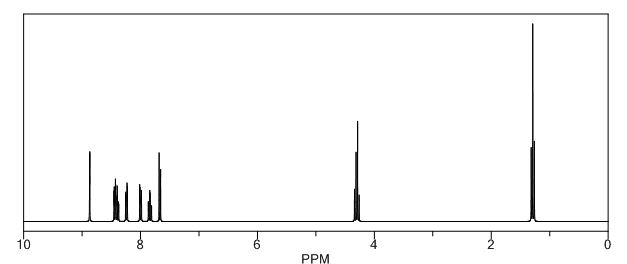
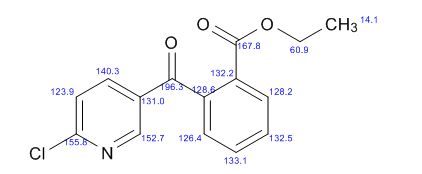
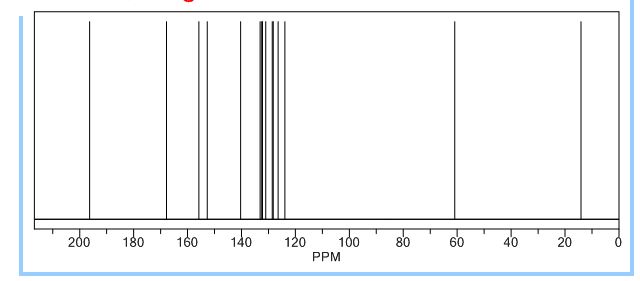
//////
O=C(c1cnc(Cl)cc1)c2ccccc2C(=O)OCC






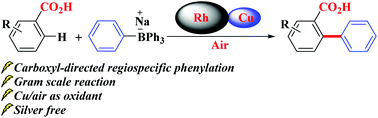








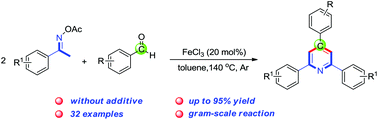



 New class of cyclic Weinreb amides for oxidative C-H
olefination or Heck reaction
New class of cyclic Weinreb amides for oxidative C-H
olefination or Heck reaction









