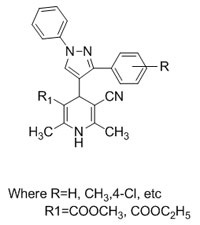
I am happy to share with you our laboratory recent publication on asymmetric catalysis.
This communication deals with the understanding and development of Brønsted Acid-Primary Amine as a Synergistic-catalyst for Stereoselective Asymmetric Barbas [4+2]-cycloaddition reaction by using 2-aminobuta-1,3-diene-cata
I believe this reaction can become good tool for chemists.
I congratulate my co-workers for their hard work to make this work more successful.
For more information, please click below link:
http://
This communication deals with the understanding and development of Brønsted Acid-Primary Amine as a Synergistic-catalyst for Stereoselective Asymmetric Barbas [4+2]-cycloaddition reaction by using 2-aminobuta-1,3-diene-cata
I believe this reaction can become good tool for chemists.
I congratulate my co-workers for their hard work to make this work more successful.
For more information, please click below link:
http://
(1R,3S,5S)-5-hydroxy-3-methyl-2'-oxospiro[cyclohexane-1,3'-indoline]-2,2-dicarbonitrile (7ag): Prepared by following the
procedure E and purified by column chromatography using EtOAc/hexane and isolated as solid.
[]D
25
= –119.2 (c
= 0.1 g/100 mL, CHCl3, 99% ee);
IR (Neat): max 3313, 2977, 2930, 1701, 1618, 1479, 1350, 1272, 1246, 1200,
1148, 1066, 1014 and 750 cm-1
;
1H NMR (CDCl3) 8.58 (1H, br s, NH), 7.64 (1H, d, J = 8.0 Hz), 7.41 (1H, t, J = 7.5
Hz), 7.23 (1H, t, J = 7.5 Hz), 6.99 (1H, d, J = 7.5 Hz), 5.32 (1H, dd, J = 11.0, 4.0 Hz), 4.22-4.18 (1H, m), 3.44-3.89
(1H, m), 2.33 (1H, dd, J = 16.0, 4.0 Hz), 2.20-2.13 (2H, m), 1.89 (1H, dt, J = 13.0, 3.5 Hz), 1.40 (3H, d, J = 6.5 Hz);
13C NMR
S-15
(CDCl3, DEPT-135) 178.2 (C, N_C=O), 139.8 (C), 130.9 (CH), 127.6 (C), 124.9 (CH), 124.2 (CH), 112.9 (C, CN), 111.8 (C, CN),
110.8 (CH), 64.7 (CH), 52.3 (C), 47.2 (C), 36.9 (CH2), 34.0 (CH2), 28.8 (CH), 18.4 (CH3);
HRMS m/z 304.1060 (M + Na), calcd for
C16H15N3O2Na 304.1062.
Ramachary D B
- Details

Dr. D. B. Ramchary, Ph.D. Associate Professor
School of Chemistry
University of Hyderabad
Prof. C. R. Rao Road, Gachibowli
Hyderabad, 500046, India
Tel: 0091-40-23134816
Email: ramsc@uohyd.ernet.in
School of Chemistry
University of Hyderabad
Prof. C. R. Rao Road, Gachibowli
Hyderabad, 500046, India
Tel: 0091-40-23134816
Email: ramsc@uohyd.ernet.in
Ramachary obtained his Masters degree (General Chemistry) at the University of Hyderabad in 1996. There as part of curricula he studied and actively involved in the project, entitled Synthesis and Utilization of Aromatic Radical Anions for Reduction of Carbon Monoxide (with Prof. M. Periasamy).
Ramachary obtained his Doctoral degree (Organic Synthesis) at the Indian Institute of Science, Bangalore for his research work on Total Synthesis of Sesquiterpenes Containing Three Contiguous Quaternary Carbon Atoms (with Prof. A. Srikrishna).
In January 2002, he moved to Prof. Carlos F. Barabas III research group as Skaggs Post Doctoral fellow at The Scripps Research Institute, San Diego. After three year post doctoral studies at TSRI, he then joined as Faculty member in School of Chemistry, University of Hyderabad in Jan 2005, where at present he is a Reader.
Dr Ramachary has been awarded the INSA Medal for Young Scientists in Chemical Sciences for the year 2006 for his outstanding contributions to the emerging area of asymmetric organocatalysis.
The main focus of his research group is to engineer the novel and green asymmetric cascade and multi-component reactions (MCRs) to generate the biologically important molecules and natural products in a single step via emerging chiral amines or amino acid-catalysis.
His research group is actively engaged in the design and synthesis of novel enzyme mimetic small organic amines and amino acids to catalyze the fundamental organic reactions in enantioselective manner.
His interest about inquisitive questions like how small can be highly active and stereo-selective catalysts and what are the minimal functional and structural features required in a chiral catalyst had made him to venture in the most advanced versions of synthetic chemistry.
Ramachary obtained his Doctoral degree (Organic Synthesis) at the Indian Institute of Science, Bangalore for his research work on Total Synthesis of Sesquiterpenes Containing Three Contiguous Quaternary Carbon Atoms (with Prof. A. Srikrishna).
In January 2002, he moved to Prof. Carlos F. Barabas III research group as Skaggs Post Doctoral fellow at The Scripps Research Institute, San Diego. After three year post doctoral studies at TSRI, he then joined as Faculty member in School of Chemistry, University of Hyderabad in Jan 2005, where at present he is a Reader.
Dr Ramachary has been awarded the INSA Medal for Young Scientists in Chemical Sciences for the year 2006 for his outstanding contributions to the emerging area of asymmetric organocatalysis.
The main focus of his research group is to engineer the novel and green asymmetric cascade and multi-component reactions (MCRs) to generate the biologically important molecules and natural products in a single step via emerging chiral amines or amino acid-catalysis.
His research group is actively engaged in the design and synthesis of novel enzyme mimetic small organic amines and amino acids to catalyze the fundamental organic reactions in enantioselective manner.
His interest about inquisitive questions like how small can be highly active and stereo-selective catalysts and what are the minimal functional and structural features required in a chiral catalyst had made him to venture in the most advanced versions of synthetic chemistry.
For the more information about his research group please Click here for Home Page
Significant Publications:
D. B. Ramachary, M. Kishor and Y. Vijayendar Reddy, Development of Pharmaceutical Drugs, Drug Intermediates and Ingredients by Using Direct Organo-Click Reactions, Eur. J. Org. Chem., 2007, xxxx-xxxx (DOI: 10.1002/ejoc.200701014).
D. B. Ramachary, G. Babul Reddy and Rumpa Mondal, New Organocatalyst for Friedel-Crafts Alkylation of 2-Naphthols with Isatins: Application of an Organo-Click Strategy for the Cascade Synthesis of Highly Functionalized Molecules, Tetrahedron Lett., 2007, 48, 7618-7623.
D. B. Ramachary and M. Kishor, Organocatalytic Sequential One-Pot Double Cascade Asymmetric Synthesis of Wieland-Miescher Ketone Analogs from a Knoevenagel/Hydrogenation/Robinson Annulation Sequence: Scope and Applications of Organocatalytic Bio-Mimetic Reductions, J. Org. Chem., 2007, 72, 5056-5068.
D. B. Ramachary, K. Ramakumar and V. V. Narayana, Organocatalytic Cascade Reactions Based on Push-Pull Dienamine Platform: Synthesis of Highly Substituted Anilines, J. Org. Chem., 2007, 72, 1458-1463.
D. B. Ramachary and G. Babul Reddy, Towards Organo-Click Reactions: Development of Pharmaceutical Ingredients by Using Direct Organocatalytic Bio-Mimetic Reductions, Org. Biomol. Chem., 2006, 4, 4463-4468.
D. B. Ramachary and Rumpa Mondal, Direct Organocatalytic Hydroalkoxylation of a,b-Unsaturated Ketones, Tetrahedron Lett., 2006, 47, 7689-7693.
Jorly Joseph, D. B. Ramachary, Eluvathingal D. Jemmis, Electrostatic Repulsion as an additional Selectivity Factor in Asymmetric Proline Catalysis, Org. Biomol. Chem., 2006, 4, 2685-2689.
D. B. Ramachary, M. Kishor and G. Babul Reddy, Development of Drug Intermediates by Using Direct Organocatalytic Multi-Component Reactions, Org. Biomol. Chem., 2006, 4, 1641-1646.
D. B. Ramachary, M. Kishor and K. Ramakumar, A Novel and Green Protocol for Two-Carbon Homologation: A Direct Amino Acid/K2CO3-Catalyzed Four-Component Reaction of Aldehydes, Active Methylenes, Hantzsch Esters and Alkyl Halides, Tetrahedron Lett., 2006, 47, 651-656.
D. B. Ramachary, K. Ramakumar and M. Kishor, Direct Organocatalytic in situ Generation of Novel Push-Pull Dienamines: Application in Tandem Claisen-Schmidt/Iso-Aromatization Reactions, Tetrahedron Lett., 2005, 46, 7037-7042.
D. B. Ramachary and Carlos F. Barbas III, Direct Amino Acid-Catalyzed Asymmetric Desymmetrization of meso-Compounds: Tandem Aminoxylation/O-N Bond Heterolysis Reactions, Org. Lett., 2005, 7, 1577-1580.
D. B. Ramachary, Naidu S. Chowdari and Carlos F. Barbas III, Organocatalytic Asymmetric Domino Knoevenagel/Diels-Alder Reactions: A Bioorganic Approach to the Diastereospecific and Enantioselective Construction of Highly Substituted Spiro[5,5]undecane-1,5,9-triones, Angew. Chem. Int. Ed., 2003, 42, 4233-4237.
D. B. Ramachary, M. Kishor and Y. Vijayendar Reddy, Development of Pharmaceutical Drugs, Drug Intermediates and Ingredients by Using Direct Organo-Click Reactions, Eur. J. Org. Chem., 2007, xxxx-xxxx (DOI: 10.1002/ejoc.200701014).
D. B. Ramachary, G. Babul Reddy and Rumpa Mondal, New Organocatalyst for Friedel-Crafts Alkylation of 2-Naphthols with Isatins: Application of an Organo-Click Strategy for the Cascade Synthesis of Highly Functionalized Molecules, Tetrahedron Lett., 2007, 48, 7618-7623.
D. B. Ramachary and M. Kishor, Organocatalytic Sequential One-Pot Double Cascade Asymmetric Synthesis of Wieland-Miescher Ketone Analogs from a Knoevenagel/Hydrogenation/Robinson Annulation Sequence: Scope and Applications of Organocatalytic Bio-Mimetic Reductions, J. Org. Chem., 2007, 72, 5056-5068.
D. B. Ramachary, K. Ramakumar and V. V. Narayana, Organocatalytic Cascade Reactions Based on Push-Pull Dienamine Platform: Synthesis of Highly Substituted Anilines, J. Org. Chem., 2007, 72, 1458-1463.
D. B. Ramachary and G. Babul Reddy, Towards Organo-Click Reactions: Development of Pharmaceutical Ingredients by Using Direct Organocatalytic Bio-Mimetic Reductions, Org. Biomol. Chem., 2006, 4, 4463-4468.
D. B. Ramachary and Rumpa Mondal, Direct Organocatalytic Hydroalkoxylation of a,b-Unsaturated Ketones, Tetrahedron Lett., 2006, 47, 7689-7693.
Jorly Joseph, D. B. Ramachary, Eluvathingal D. Jemmis, Electrostatic Repulsion as an additional Selectivity Factor in Asymmetric Proline Catalysis, Org. Biomol. Chem., 2006, 4, 2685-2689.
D. B. Ramachary, M. Kishor and G. Babul Reddy, Development of Drug Intermediates by Using Direct Organocatalytic Multi-Component Reactions, Org. Biomol. Chem., 2006, 4, 1641-1646.
D. B. Ramachary, M. Kishor and K. Ramakumar, A Novel and Green Protocol for Two-Carbon Homologation: A Direct Amino Acid/K2CO3-Catalyzed Four-Component Reaction of Aldehydes, Active Methylenes, Hantzsch Esters and Alkyl Halides, Tetrahedron Lett., 2006, 47, 651-656.
D. B. Ramachary, K. Ramakumar and M. Kishor, Direct Organocatalytic in situ Generation of Novel Push-Pull Dienamines: Application in Tandem Claisen-Schmidt/Iso-Aromatization Reactions, Tetrahedron Lett., 2005, 46, 7037-7042.
D. B. Ramachary and Carlos F. Barbas III, Direct Amino Acid-Catalyzed Asymmetric Desymmetrization of meso-Compounds: Tandem Aminoxylation/O-N Bond Heterolysis Reactions, Org. Lett., 2005, 7, 1577-1580.
D. B. Ramachary, Naidu S. Chowdari and Carlos F. Barbas III, Organocatalytic Asymmetric Domino Knoevenagel/Diels-Alder Reactions: A Bioorganic Approach to the Diastereospecific and Enantioselective Construction of Highly Substituted Spiro[5,5]undecane-1,5,9-triones, Angew. Chem. Int. Ed., 2003, 42, 4233-4237.
//////



















 A green chemistry approach to activating alcohols
A green chemistry approach to activating alcohols
 July-August 2012, Volume 2, No.4
July-August 2012, Volume 2, No.4