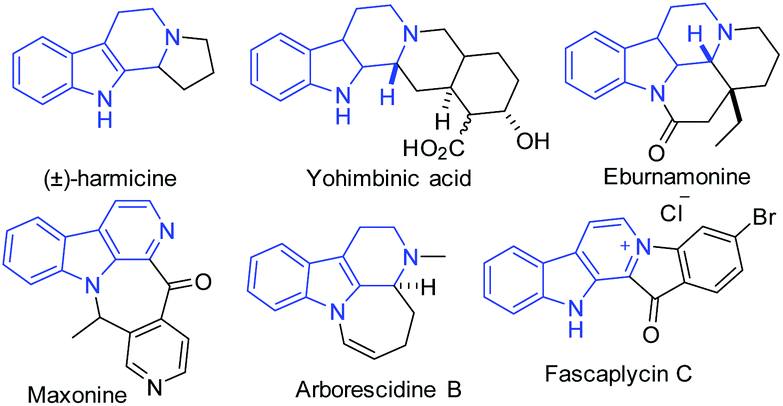
A new rearranged spongian diterpene, darwinolide, has been isolated from the Antarctic Dendroceratid sponge Dendrilla membranosa.
Characterized on the basis of spectroscopic and crystallographic
analysis, the central seven-membered ring is hypothesized to originate
from a ring-expansion of a spongian precursor. Darwinolide displays
4-fold selectivity against the biofilm phase of methicillin-resistant Staphylococcus aureus
compared to the planktonic phase and may provide a scaffold for the
development of therapeutics for this difficult to treat infection.

NMR Data for Darwinolide (CDCl3)
| position | δC, typea | δH (J in Hz)b | COSY | HMBC | ROESY |
|---|---|---|---|---|---|
| 1a | 38.6, CH2 | 1.08, m | 1b,2a,2b | 2,3,4,9,18,19,20 | |
| b | 1.54, m | 1a,2a,2b | 2,3,4,5,10,20 | ||
| 2a | 18.7, CH2 | 1.50, m | 1b,3a,3b | 1,4,10 | |
| b | 1.59, m | 1a,1b,3b | 4 | ||
| 3a | 39.2, CH2 | 1.11, m | 2a | 2,4,18,19 | |
| b | 1.37, m | 2a,2b | 4,10,18,19 | ||
| 4 | 30.8, C | ||||
| 5a | 50.5, CH2 | 1.08, d (14.1) | 5b | 3,4,9,18,19,20 | |
| b | 1.38, d (14.1) | 5a | 1,2,3,4,11,18,19,20 | ||
| 6 | 15.6, CH3 | 2.39, d (2.3) | 14 | 7,8,9,13,17 | 20 |
| 7 | 119.5, C | ||||
| 8 | 159.5, C | ||||
| 9 | 57.3, CH | 2.08, m | 11a,11b | 1,5,6,7,8,10,11,12,20 | 14 |
| 10 | 36.0, C | ||||
| 11a | 19.2, CH2 | 1.42, m | 9,12b | 8,9,10,12,13,16 | |
| b | 1.64, m | 9,12a,12b | 8,9,12,13 | ||
| 12a | 25.6, CH2 | 1.92, m | 11b,13 | 9,13,14,16 | |
| b | 1.19, m | 11a,11b | 13,14,16 | ||
| 13 | 43.2, CH | 2.24, m | 12a,14,16 | 12,16 | |
| 14 | 45.1, CH | 3.93, tt (7.0, 2.4) | 6,13,15 | 7 | 9,13,15 |
| 15 | 103.9, CH | 6.07, d (7.0) | 14 | 7,14,17 | 14 |
| 16 | 103.8, CH | 5.93, s | 13 | 12,13,14,15,21 | 12a |
| 17 | 167.7, C | ||||
| 18 | 33.9, CH3 | 0.86, s | 19 | 2,3,4,5,10,19 | 19 |
| 19 | 28.5, CH3 | 0.98, s | 18 | 3,4,5,10,18 | 18 |
| 20 | 22.1, CH3 | 1.14, s | 1,5,9,10 | 6 | |
| 21 | 169.7, C | ||||
| 22 | 21.2, CH3 | 2.08, s | 16,21 |
a
Recorded at 125 MHz.
b
Recorded at 500 MHz.

cosy
 .
.
roesyad
Darwinolide, a New Diterpene Scaffold That Inhibits Methicillin-Resistant Staphylococcus aureus Biofilm from the Antarctic Sponge Dendrilla membranosa
† Department
of Chemistry, University of South Florida, 4202 East Fowler Avenue, CHE205, Tampa, Florida 33620, United States
‡ Center
for Drug Discovery and Innovation, University
of South Florida, 3720
Spectrum Boulevard, Suite 303, Tampa, Florida 33612, United States
§ Department
of Cell Biology, Microbiology and Molecular Biology, University of South Florida, 4202 East Fowler Avenue, ISA2015, Tampa, Florida 33620, United States
∥ Department
of Biology, University of Alabama at Birmingham, Birmingham, Alabama 35294, United States
Org. Lett., 2016, 18 (11), pp 2596–2599
DOI: 10.1021/acs.orglett.6b00979
http://pubs.acs.org/doi/full/10.1021/acs.orglett.6b00979
*E-mail: bjbaker@usf.edu.
/////////





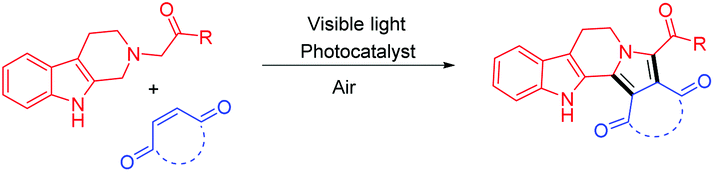
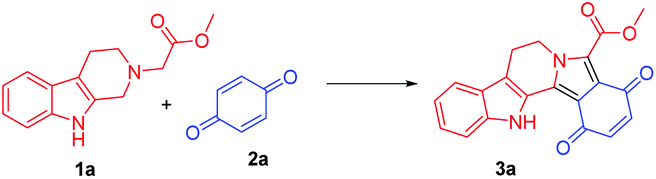
![Graphical abstract: Visible-light photoredox catalysis: direct synthesis of fused β-carbolines through an oxidation/[3 + 2] cycloaddition/oxidative aromatization reaction cascade in batch and flow microreactors](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C5QO00207A) .
.