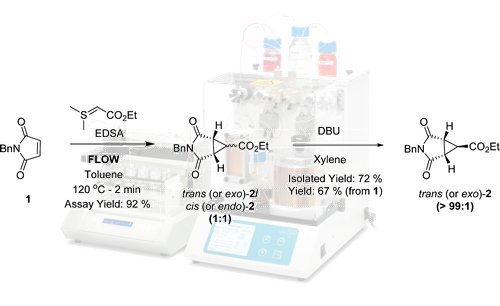
![Synthetic Scheme for 1,2,4-triazolo[3,4-b][1,3,4]thiadiazine bearing substituted ...](http://ars.els-cdn.com/content/image/1-s2.0-S187853521500218X-gr2.jpg)
- Scheme 1.Synthetic Scheme for 1,2,4-triazolo[3,4-b][1,3,4]thiadiazine bearing substituted phenylquinolin-2-one (4a–4t). Where R = H, 2-Br, 3-Br, 4-Br, 2-Cl, 3-Cl, 4-Cl, 2-F, 3-F, 4-F, 2-NO2, 3-NO2, 4-NO2, 2-CH3, 3-CH3, 4-CH3, 2-OCH3, 3-OCH3, 4-OCH3, 3,4,5-t-OCH3. Reagents and conditions: (a) C2H5OH, Nitrobenzene, Con. H2SO4, reflux, 80 °C, 4 h; (b) chloroacetic acid, NaHCO3, water, CuO, reflux, 80 °C, 5 h; (c) thiocarbohydrazide, Δ, 2 h; (d) phenacyl bromide, anhydrous ethanol, NH4OH, 50 °C, 6 h.
 C and C
C and C S
S C stretching vibrations respectively and no absorption peaks at 3140–3280 cm−1, 3050–3090 cm−1 due to -NH2 and -SH groups respectively indicated smooth cyclization of triazoles leading to the formation of thiadiazine ring. Further, 1H NMR spectra of the synthesized compounds were confirmed by the appearance of S
C stretching vibrations respectively and no absorption peaks at 3140–3280 cm−1, 3050–3090 cm−1 due to -NH2 and -SH groups respectively indicated smooth cyclization of triazoles leading to the formation of thiadiazine ring. Further, 1H NMR spectra of the synthesized compounds were confirmed by the appearance of S CH2 proton of 1,3,4-thiadiazine ring at 4.40–4.54 ppm and
CH2 proton of 1,3,4-thiadiazine ring at 4.40–4.54 ppm and  CH2
proton at 5.97–6.20 ppm as broad singlet. All other aromatic protons
were observed at expected region. Mass spectra (ESI-MS) of all the
synthesized compounds showed molecular ion [M + H]+ peak in agreement with their molecular formula. Physical and elemental analyses are listed in Table 1.
CH2
proton at 5.97–6.20 ppm as broad singlet. All other aromatic protons
were observed at expected region. Mass spectra (ESI-MS) of all the
synthesized compounds showed molecular ion [M + H]+ peak in agreement with their molecular formula. Physical and elemental analyses are listed in Table 1.2.6.20. 6,7,8-Trimethoxy-4-phenyl-1-({6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl}methyl)-1,2-dihydroquinolin-2-one (4t)
White needle Crystal; IR (KBr, cm−1): 3023 (Ar C H str), 2901 (Aliphatic C
H str), 2901 (Aliphatic C H str),1706 (C
H str),1706 (C O str), 1620 (C
O str), 1620 (C N str), 1564 (C
N str), 1564 (C C str), 1330 (Ar C
C str), 1330 (Ar C N str), 683 (C
N str), 683 (C S
S C); 1H NMR (CDCl3, 300 MHz): δ (ppm): 3.84 (s, 9H, 3xOCH3), 4.51 (s, 2H, S
C); 1H NMR (CDCl3, 300 MHz): δ (ppm): 3.84 (s, 9H, 3xOCH3), 4.51 (s, 2H, S CH2
CH2 ), 5.98 (s, 2H, CH2), 6.85 (s, 1H, CH
), 5.98 (s, 2H, CH2), 6.85 (s, 1H, CH ), 7.45(d, 2H, J = 8.5 Hz, Ar
), 7.45(d, 2H, J = 8.5 Hz, Ar H), 7.48(m,5H, Ar
H), 7.48(m,5H, Ar H), 7.74 (q, 4H, Ar
H), 7.74 (q, 4H, Ar H). ESI-MS (m/z) calcd. is 539.6, found 540.7 [M + H]+; anal. calcd. for C29H25N5O4S (539.60): C 64.55, H 4.67, N 12.98; found: C 64.54, H 4.63, N 12.95.
H). ESI-MS (m/z) calcd. is 539.6, found 540.7 [M + H]+; anal. calcd. for C29H25N5O4S (539.60): C 64.55, H 4.67, N 12.98; found: C 64.54, H 4.63, N 12.95.
 H str), 2901 (Aliphatic C
H str), 2901 (Aliphatic C H str),1706 (C
H str),1706 (C O str), 1620 (C
O str), 1620 (C N str), 1564 (C
N str), 1564 (C C str), 1330 (Ar C
C str), 1330 (Ar C N str), 683 (C
N str), 683 (C S
S C); 1H NMR (CDCl3, 300 MHz): δ (ppm): 3.84 (s, 9H, 3xOCH3), 4.51 (s, 2H, S
C); 1H NMR (CDCl3, 300 MHz): δ (ppm): 3.84 (s, 9H, 3xOCH3), 4.51 (s, 2H, S CH2
CH2 ), 5.98 (s, 2H, CH2), 6.85 (s, 1H, CH
), 5.98 (s, 2H, CH2), 6.85 (s, 1H, CH ), 7.45(d, 2H, J = 8.5 Hz, Ar
), 7.45(d, 2H, J = 8.5 Hz, Ar H), 7.48(m,5H, Ar
H), 7.48(m,5H, Ar H), 7.74 (q, 4H, Ar
H), 7.74 (q, 4H, Ar H). ESI-MS (m/z) calcd. is 539.6, found 540.7 [M + H]+; anal. calcd. for C29H25N5O4S (539.60): C 64.55, H 4.67, N 12.98; found: C 64.54, H 4.63, N 12.95.
H). ESI-MS (m/z) calcd. is 539.6, found 540.7 [M + H]+; anal. calcd. for C29H25N5O4S (539.60): C 64.55, H 4.67, N 12.98; found: C 64.54, H 4.63, N 12.95.Arabian Journal of Chemistry

Available online 11 July 2015
ORIGINAL ARTICLE
Synthesis, characterization and antimicrobial evaluation of some novel 1,2,4-triazolo[3,4-b][1,3,4]thiadiazine bearing substituted phenylquinolin-2-one moiety
- a Department of Pharmaceutical Sciences, Faculty of Health Sciences, Allahabad, Uttar Pradesh 211007, India
- b Department of Pharmaceutical Chemistry, United Institute of Pharmacy, UPSIDC, Naini, Allahabad, Uttar Pradesh 211010, India
- Received 3 April 2014, Accepted 7 July 2015, Available online 11 July 2015
- Open Access funded by King Saud University
- Under a Creative Commons license

 .
.