Synthesis and Reactions of Alkenes:
'via Blog this'
Organic Synthesis International by Dr Anthony Melvin Crasto Ph.D, Worlddrugtracker, Million hits on google on all sites, One lakh connections worldwide. Pushing boundaries.Interaction site for Organic chemists worldwide, Mail me at amcrasto@gmail.com if you like me
Pages
- Home
- THESIS
- orgsyn.org site
- Chemspider synthetic pages
- ABOUT ME
- DISCLAIMER
- Montelukast and similar drugs
- Glitazar and glitazone series
- ABAN SERIES
- melteon series
- USEFUL LINKS
- LITERATURE SEARCH
- DIPINE SERIES
- International spaces
- GLIPTIN SERIES 2/2
- Atom Economy (AE), Reaction Mass Efficiency (RME) ...
Monday, 9 September 2013
OMICS Publishing Group | Full-text | Organosilicon Chemisty: Past, Present and Future
| Organosilicon Chemisty: Past, Present and Future | |||
| Vladimir Ya. LEE* | |||
| Department of Chemistry, University of Tsukuba, Japan | |||
| |||
OMICS Publishing Group | Full-text | Organosilicon Chemisty: Past, Present and Future:
'via Blog this'
Sunday, 8 September 2013
A Facile One-Pot Synthesis of 3,5-Disubstituted Isoxazole Derivatives Using Hydroxy (Tosyloxy) Iodobenzene - Jadhav - 2013 - Journal of Heterocyclic Chemistry - Wiley Online Library
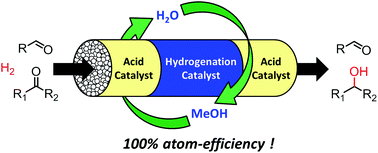
Highly atom-efficient and chemoselective reduction of ketones in the presence of aldehydes using heterogeneous catalysts - Green Chemistry (RSC Publishing)
Friday, 6 September 2013
Selecting with Sulfur
Selecting with Sulfur

A method that selects between two products operates simply by changing the oxidation state of sulfur
Wednesday, 21 August 2013
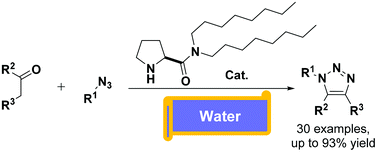
Organocatalytic 1,3-dipolar cycloaddition reactions of ketones and azides with water as a solvent
Green Chem., 2013, 15,2384-2388
DOI: 10.1039/C3GC41126E, Communication
DOI: 10.1039/C3GC41126E, Communication
*
Corresponding authors
a
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore
E-mail: chmwangj@nus.edu.sg ;
Fax: (+)65-6516-1691
E-mail: chmwangj@nus.edu.sg ;
Fax: (+)65-6516-1691
b
HuBei Collaborative Innovation Center of Non-power Nuclear Technology, Hubei University of Science and Technology, Hubei Province, China
First published online 22 Jul 2013
We reported an enamine catalyzed strategy to fully promote a 1,3-dipolar cycloaddition to access a vast pool of substituted 1,2,3-triazoles in water.
Subscribe to:
Comments (Atom)