
Otto Diels and his pupil Kurt Alder received the Nobel Prize in 1950 for their discovery and work on the reaction that bears their names. Its great usefulness lies in its high yield and high sterospecificity. The Diels-Alder reaction has been employed extensively in the synthesis of complex natural products because it is possible to exploit the formation of up to four asymmetric carbons in one reaction and at the same time it is also possible to control the regioselectivity of the reaction.

Many chemical reactions are done under reflux. By refluxing a reaction in a particular solvent, the reaction is kept at a constant temperature in a constant state of mixing. The boiling point of the solvent determines the reaction temperature. The solvent is boiled out of the reaction solution but quickly condenses and returns to the flask once the solvent vapors enter the reflux condenser.

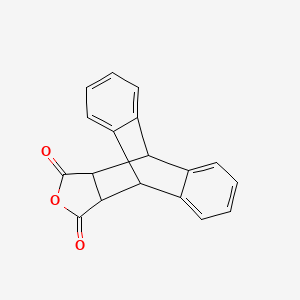
Anthracene, 2,5-furandione adduct, 17-oxapentacyclo[6.6.5.0~2,7~.0~9,14~.0~15,19~]nonadeca-2,4,6,9(14),10,12-hexaene-16,18-dione, 5443-16-3, Anthracene,maleic anhydride adduct, AGN-PC-00GKYN, AC1L3CY7
Molecular Formula: C18H12O3
Molecular Weight: 276.28608 g/mol
Reference: “Experiments in Organic Chemistry” 2nd ed. (2000)
R.K. Hill & J. Barbaro,
Contemporary Publishing Company of Raleigh, Inc.
We will be performing the Diels Alder reaction with a very popular dieneophile – maleic anhydride. The diene we will be using is anthracene - a polycylic aromatic hydrocarbon (PAH) that can be found in coal, petroleum, and charcoal grilled hamburgers. The interesting aspect of anthracene is that it offers several different diene sites for the Diels Alder addition of maleic anhydride.
Place 3g of pure anthracene, 30 ml of dry xylene (See Note ) and 1.5 g of maleic anhydride in a 100 ml dry round bottomed flask. Attach a water condenser and reflux the contents on a water-bath for 25 min with frequent shaking.Cool the mixture and collect the solid on a Buchner funnel. Recrystallize the adduct from ethyl acetate. The yield is 3.3 g, m.p.  C.
C.

Procedure:
A. Reaction and Isolation of Products
- Add 2 g of anthracene to a 100 or 250mL round bottom flask.
- Add 1 g of maleic anhydride to the round bottom. Despite its benign appearance, solid maleic anhydride is toxic and should not be handled with bare hands.
- Add 25 mL of xylene (dimethyl benzene) to the round bottom. Do not remove the xylene bottle from the fume hood. Do this operation in the hood!
- Add three boiling chips to the round bottom. Swirl the reactants to mix. The solids will not totally dissolve at this time.
- Attach the round bottom to a reflux condenser mounted on a ring stand. Attach water hoses as shown in the preceding diagram
- Use a thermwell mounted on an iron ring to heat the solution. Heat the mixture to boiling, and then adjust the heat input so that the boiling is maintained at a steady rate.
- Reflux for 25 minutes. Note any changes in the reaction mixture. Complement your “hood mate” on how nice he/she looks today.
- 8. Turn off the heat source. Carefully transferring the hot contents to a 50 or 150 mL beaker at this time will make to easier to remove the crystals that form.
- 9. Chill in an ice bath.
- 10. Vacuum filter the solid (crude) product in a vacuum (Buchner) funnel. You may wash it with a small amount of ice-cold xylene.
- 11. Remove boiling chips with a tweezers.
- 12. Admire and the crude product.
- B. Recrystallization
- Recrystallize your crude product with hexane.
The Diels-Alder reaction is a cycloaddition reaction, a reaction in which two molecules undergo addition to yield a cyclic product. Various types of cycloaddition reaction are known. Because a Diels-Alder reaction involves two double bonds(four π electrons) of a conjugated diene and one double bond (two π electrons) of the other reactant, this type of reaction is called [4+2] cycloaddition. In a Diels-Alder reaction, the conjugated diene is referred to as the diene; the other compound is called the dienopile.

[4+2] cycloaddition
This Diels-Alder reaction is carried out by boiling the reactants in xylene. Both reactants are soluble in xylene, and the reaction is rapid because of the high boilig point of the solvent, xylene. As the mixture cools, the product crystallizes. The product is isolated by filtration; if the starting anthracene was pure, it requires no further purification.
It is difficult to remove the high-boiling xylene by “air-drying” the crystals. In addition, exposure to the air results in the hydrolysis of anhydrides by atmospheric moisture.

For this reason, the product is dried under an inverted beaker along with some paraffin wax, which dissolves xylene vapors and thus acts as a “dessicant.”
Reaction Equation

Chemicals
1. Anthracene: 1.0g

2. Maleic anhydride: 0.5g

3. Xylene: 17.5mL
Procedure
1. Place 1.0g anthracene in a 25mL round-bottomed bottle
2. Pour 17.5mL xylene into the bottle
3. Add 0.5g maleic anhydride in the solution

4. Reflux the mixture for 30 minutes.

5. After the round-bottomed bottle cools to room temperature, put the bottle in an ice bath.

It is still very hot now

6. Filter the solid by vacuum and wash the product with ice-cold petroleum ether.

7. Collect the solid in a sample bottle and put some wax films with the solid.

| Weight of anthracene | 1.000g |
| Weight of 9,10-dihydroanthracene-9,10-endo-α,β-succinic anhydride | 1.255g |
| Yield | 89.5% |
Physical constants. A table of physical constants and safety data for the chemical compounds referred to in the procedure is found on the CHEM254lab website: <http://domin.dom.edu/faculty/jbfriesen/chem254lab.htm>
| Chemical shift (d) in ppm | multiplicity |
| 48 | doublet |
| 56 | doublet |
| 125 | doublet |
| 126 | doublet |
| 143 | singlet |
| 215 | singlet |

Molten naphthalene provides an excellent solubilizing medium for poorly soluble aromatic compounds. In many cases it is more efficient than other high-boiling solvent, such as dichlorobenzene, benzonitrile, nitrobenzene and durene. A reaction of C60 with an equimolar amount of anthracene in refluxing naphthalene gives the 1:1 Diels-Alder adduct in 67% yield.
K. Komatsua, Y. Murataa, N. Sugitaa, K. Takeuchib, T.S.M. Wan (1993). "Use of naphthalene as a solvent for selective formation of the 1:1 diels-alder adduct of C60 with anthracene". Tetrahedron Letter 34 (52): 8473–8476. doi:10.1016/S0040-4039(00)61362-X.