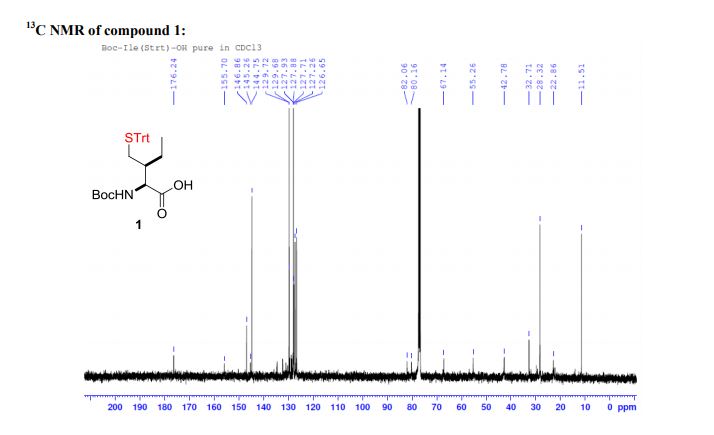
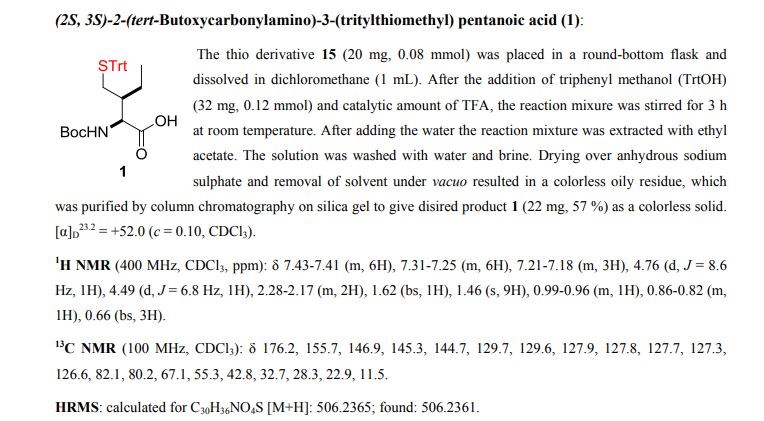
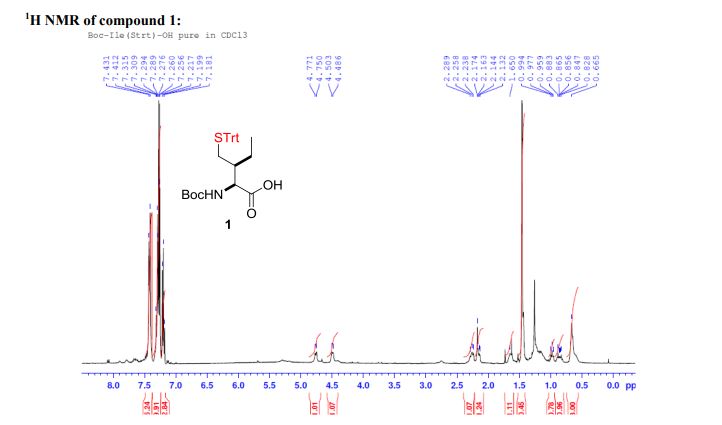
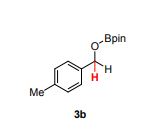
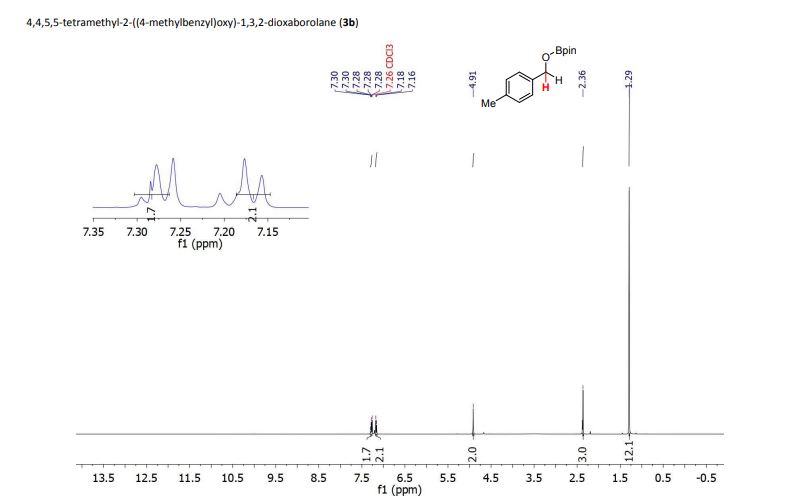
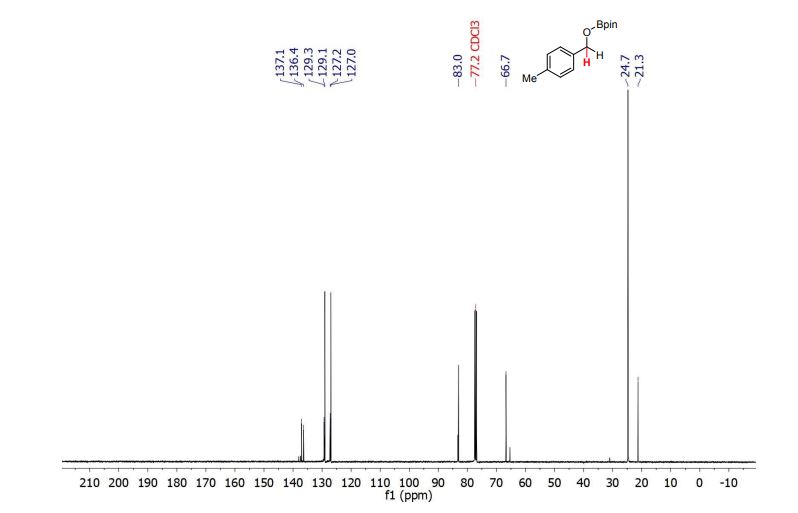
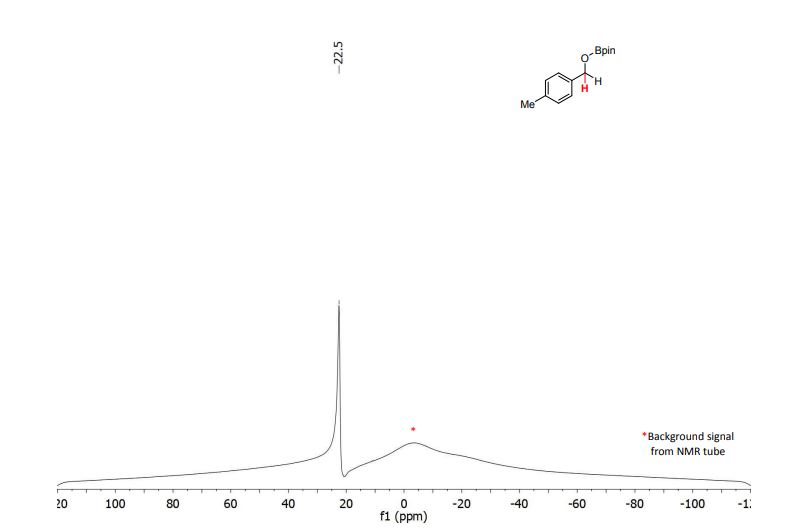
Palladium-catalyzed acetoxylation of the primary γ-C(sp3)–H bonds in the amino acids Val, Thr, and Ile was achieved using a newly discovered 5-methylisoxazole-3-carboxamide directing group. The γ-acetoxylated α-amino acid derivatives could be easily converted to γ-mercapto amino acids, which are useful for native chemical ligation (NCL). The first application of NCL at isoleucine in the semisynthesis of a Xenopus histone H3 protein was also demonstrated.
5-Methylisoxazole-3-carboxamide-Directed Palladium-Catalyzed γ-C(sp3)–H Acetoxylation and Application to the Synthesis of γ-Mercapto Amino Acids for Native Chemical Ligation
School of Biological Sciences, Nanyang Technological University, Singapore 637551
Org. Lett., 2016, 18 (11), pp 2696–2699
DOI: 10.1021/acs.orglett.6b01160
Publication Date (Web): May 24, 2016
Copyright © 2016 American Chemical Society
*E-mail: cfliu@ntu.edu.sg.
link

Research Fellow at Nanyang Technological University, Singapore
Former Post-Doctoral Fellow at Johns Hopkins School of Medicine
Studied Physical organic chemistry at PG College of Science, Saifabad/Osmania University
Studied M.P.C at Government Junior College, Hanamkonda
Studied B.Sc. at Kakatiya Government Degree College
Went to Z. P. S. S. Geesugonda
////////