Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC00313C, Communication
DOI: 10.1039/C6GC00313C, Communication
Yu Yang, Sheng Zhang, Lin Tang, Yanbin Hu, Zhenggen Zha, Zhiyong Wang
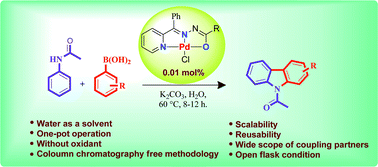
A water promoted thiolation of indoles with sulfonyl hydrazides has been developed under mild conditions in water.
A water promoted thiolation of indoles with sulfonyl hydrazides has been developed under mild conditions in water.
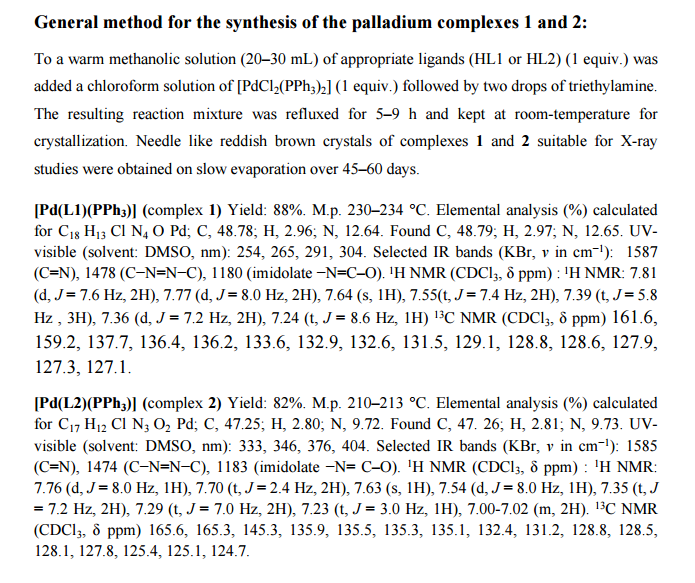
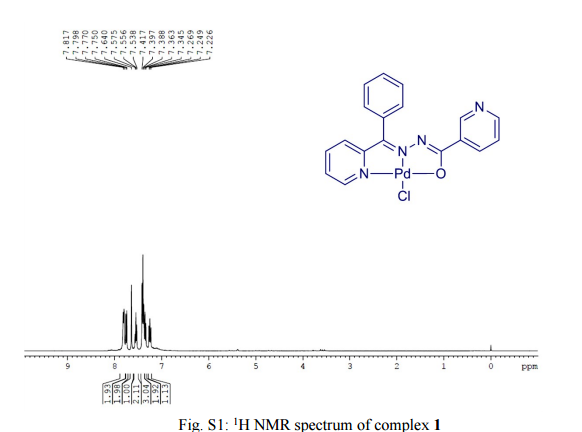
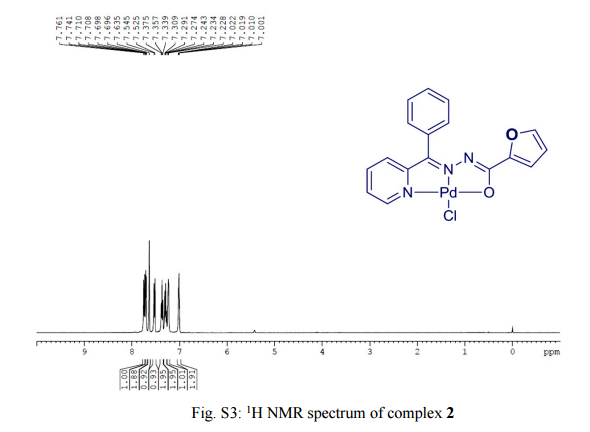
A catalyst-free thiolation of indoles with sulfonyl hydrazides was efficiently developed in water under mild conditions without any ligand or additive. The reaction provided a variety of 3-sulfenylindoles with good to excellent yields and the only by-products were nitrogen and water.
[1] F.-L. Yang, X.-T. Ma and S.-K. Tian, Chem. Eur. J., 2012, 18, 1582
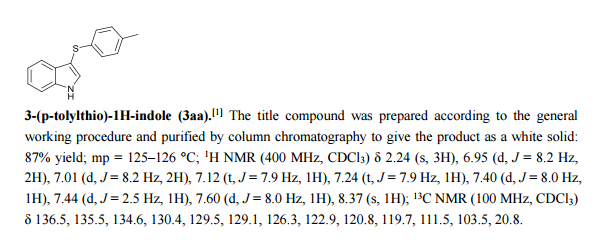
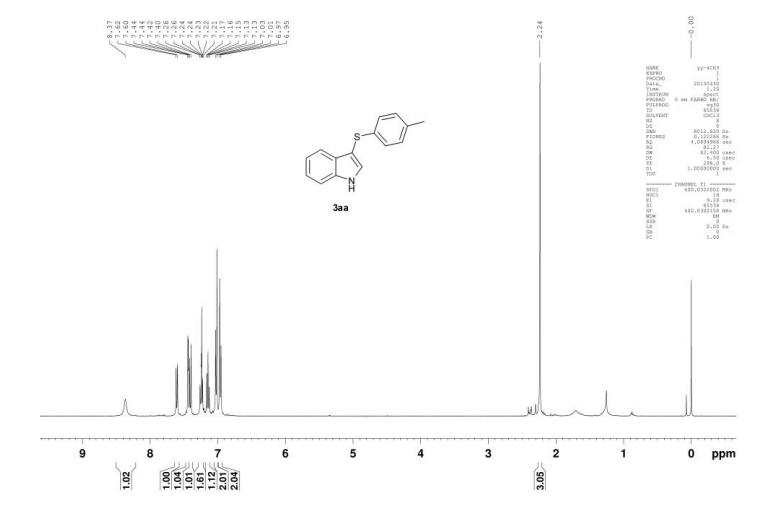
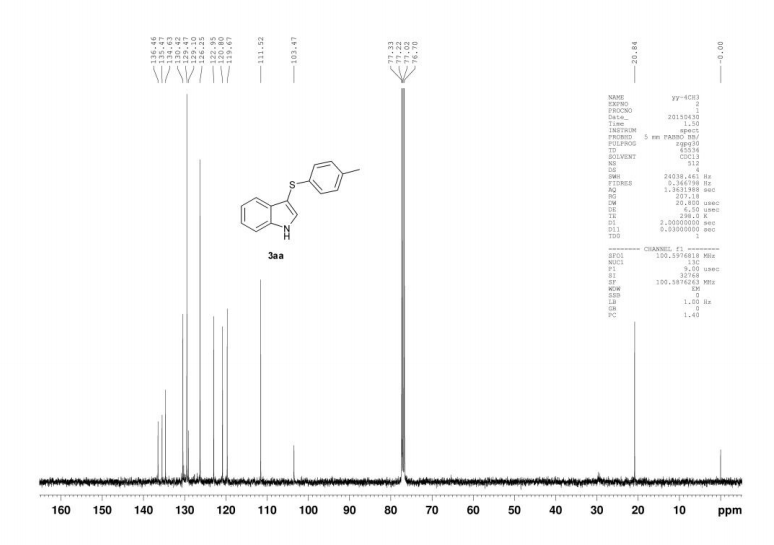
Catalyst-free thiolation of indoles with sulfonyl hydrazides for the synthesis of 3-sulfenylindoles in water
*Corresponding authors
aHefei National Laboratory for Physical Sciences at Microscale, CAS Key Laboratory of Soft Matter Chemistry and Department of Chemistry & Collaborative Innovation Center of Suzhou Nano Science and Technology, University of Science and Technology of China, Hefei, P. R. China
E-mail: zwang3@ustc.edu.cn
Fax: (+86) 551-360-3185
E-mail: zwang3@ustc.edu.cn
Fax: (+86) 551-360-3185
Green Chem., 2016, Advance Article
DOI: 10.1039/C6GC00313C
///////////

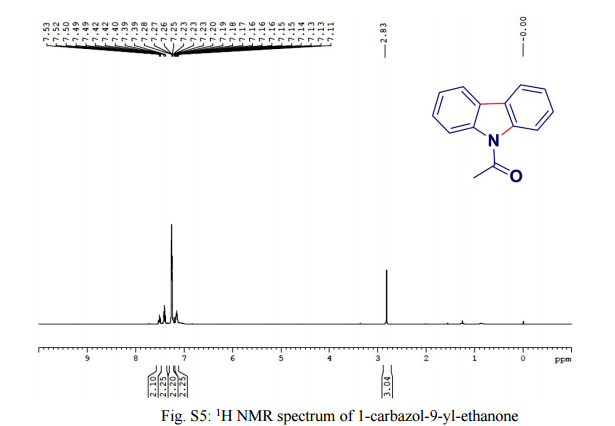
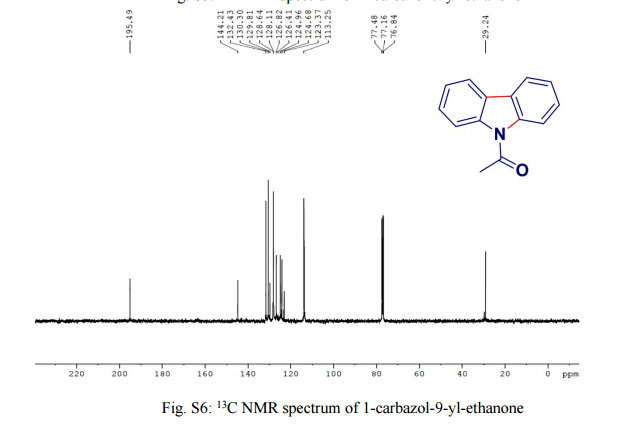








 .
.