Axay Parmar
Founder at Synthesis with Catalysts Pvt. Ltd
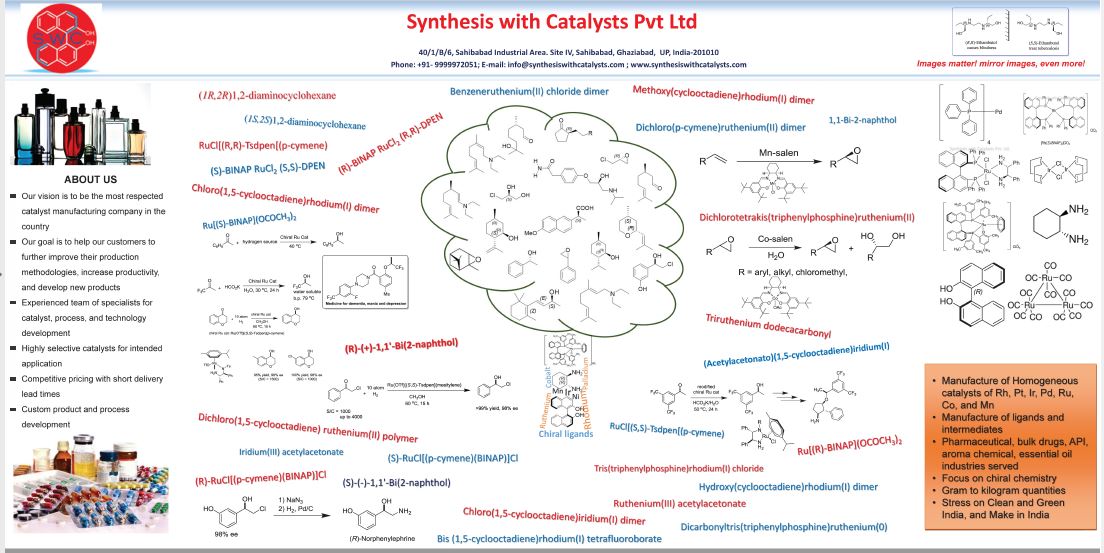
Synthesis with Catalysts Pt. Ltd. is a company started with an aim to produce chiral and achiral precious metal based catalysts on commercial scale in line with “Clean and Green India” and “Make in India” vision of Government of India. These catalysts have been developed to promote efficient, economical and environmentally benign processes for the target compounds being produced in aroma, fine chemicals and pharmaceutical industries. These catalysts and their intermediates are also extensively used in academic and industrial R&D centres across globe. In India these catalysts are currently imported at a very prohibitive cost, due to which their use is limited for want of funds. In this direction Synthesis with Catalysts Pvt. Ltd. is striving to make these products available to indigenously available at a very competitive price at small and bulk scale. We are also doing in-house research to optimize process parameters ofvarious organic transformations particularly asymmetric hydrogenation and isomerization reactionsfor customers as and when required.
For the list of our products please visit our wesitewww.synthesiswithcatalysts.com
ABOUT US
- Our vision is to be the most respected catalyst manufacturing company in the country
- Our goal is to help our customers:
- to further improve their production methodologies
- increase productivity,
- develop new products with the intervention of catalysts to make the process green and clean
- Highly selective catalysts for intended application
- Competitive pricing with short delivery lead times
- Custom product and process development
Manufacture of Homogeneous catalysts using metal ions viz. Rh, Pt, Ir, Pd, Ru, Co, and Mn
Manufacture of ligands and intermediates
Pharmaceutical, bulk drugs, API, aroma chemical, essential oil industries served
Focus on chiral chemistries
Gram to kilogram quantities
ASYMMETR
Some of the representative reactions are:
ASYMMETRIC/ CHEMOSELECTIVE HYDROGENATION CATALYSTS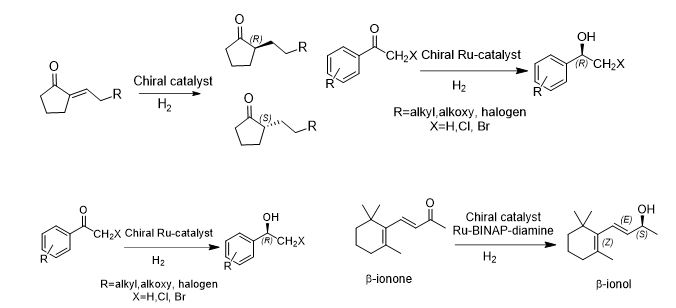
Statements
- Catalysts are chiral metal complexes derived from a precious metal ion and chiral ligands
- Ru used most frequently, Rh used in some cases to enhance chemo- and enantio- selectivity
- Chiral ligands can be selected from variety of simple and substituted BINAP alone or in combination with chiral/achiral diamines
- Suggested catalysts:
- RuCl2[(S)-BINAP](dmf)n
- RuCl2[(S)- tolBINAP][(S,S)-dpen]
- (S)-XylBINAP/(S)-DAIPEN-Ru
- (S)-XylBINAP/(S,S)-DPEN-Ru
- RuCl2[(S)-tolBINAP](pica)
- RuCl[(S,S)-TsDPEN](η6-p-cymene)
- Ru(OTf)(TsDPEN)(p-cymene)
- BINAP-Ru(II) dicarboxylate complexes
ENANTIOSELECTIVE EPOXIDATION / HKR / DKR
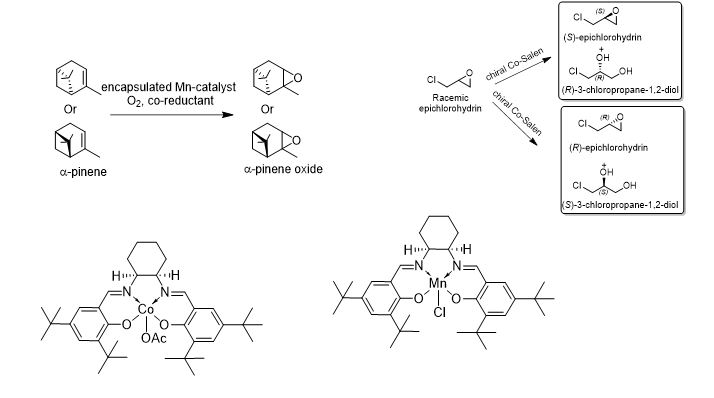
Statements:
- Transition metal complexes are used for chiral and non-chiral epoxidation of internal and prochiral olefins
- The epoxides are important intermediates for host of industrially important products
- In cases where epoxides are required in high optical purity, racemic epoxides can be subjected to Hydrolytic kinetic resolution (HKR), Aminolytic kinetic resolution (AKR), Dynamic kinetic resolutions (DKR)
- Suggested catalysts:
- Mn, Co, Cr, Al complexes of chiral SALEN ligands
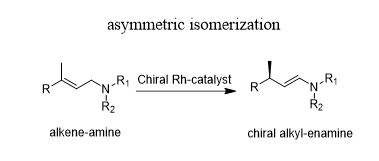
Double bond migration reactions
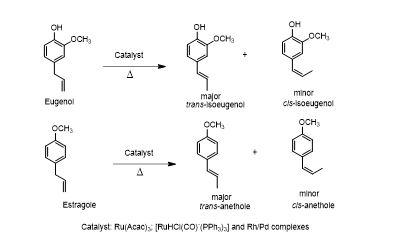
Statements:
- Rh-catalyzed asymmetric isomerization of allylic amines into the corresponding enamines is one of the most revered industrial organic transformation in asymmetric catalysis
- It has accommodated a wide range of substrates and is a key step in the industrial production of menthol
- Other industrially important isomerization is migration of terminal double bond to produce selectively trans-internal olefins
- Commercially important products like isoeugenol and trans-anetheole are produced by these transformations
- Suggested catalysts:
- Ru(acac)3
- RuHCl(CO)(PPh3)3
- Rh/Pd complexes
At Chiral India event in Mumbai where our technical director Dr. Abdi Is a speaker. With Basu Agarwal

Basu Agarwal
CEO at Synthesis with Catalysts Pvt Ltd




////////