| ||
Organic Synthesis International by Dr Anthony Melvin Crasto Ph.D, Worlddrugtracker, Million hits on google on all sites, One lakh connections worldwide. Pushing boundaries.Interaction site for Organic chemists worldwide, Mail me at amcrasto@gmail.com if you like me
Pages
- Home
- THESIS
- orgsyn.org site
- Chemspider synthetic pages
- ABOUT ME
- DISCLAIMER
- Montelukast and similar drugs
- Glitazar and glitazone series
- ABAN SERIES
- melteon series
- USEFUL LINKS
- LITERATURE SEARCH
- DIPINE SERIES
- International spaces
- GLIPTIN SERIES 2/2
- Atom Economy (AE), Reaction Mass Efficiency (RME) ...
Monday, 29 August 2016
Orthogonally Reacting Boron Coupling Reagents: A Novel Multicomponent-Multicatalytic Reaction [(MC)2R] of Dichlorovinylpyrazine
Monday, 15 August 2016
Combination of Enabling Technologies to Improve and Describe the Stereoselectivity of Wolff–Staudinger Cascade Reaction
| SYNTHESIS Highlight |
|
Synthesis
DOI: 10.1055/s-0035-1562579
DOI: 10.1055/s-0035-1562579
paper
© Georg Thieme Verlag Stuttgart · New YorkCombination of Enabling Technologies to Improve and Describe the Stereoselectivity of Wolff–Staudinger Cascade Reaction
B. Musioa, F. Mariania, b, E. P. Śliwińskia, M. A. Kabeshova, H. Odajimac, S. V. Ley*a
- aUniversity of Cambridge, Department of Chemistry, Lensfield Road, Cambridge, CB2 1EW, UK Email:svl1000@cam.ac.uk
- bUniversitat de Barcelona, Laboratori de Química Orgànica, Facultat de Farmàcia, Av. Joan XXIII s/n, 08028 Barcelona, Spain
- cSaida FDS, 143-10 Itsushiki, Yaizu-shi, Shizuoka Prefecture 4250054, Japan
Abstract
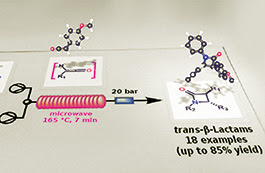
A new, single-mode bench-top resonator was evaluated for the microwave-assisted flow generation of primary ketenes by thermal decomposition of α-diazoketones at high temperature. A number of amides and β-lactams were obtained by ketene generation in situ and reaction with amines and imines, respectively, in good to excellent yields. The preferential formation of trans-configured β-lactams was observed during the [2+2] Staudinger cycloaddition of a range of ketenes with different imines under controlled reaction conditions. Some insights into the mechanism of this reaction at high temperature are reported, and a new web-based molecular viewer, which takes advantage from Augmented Reality (AR) technology, is also described for a faster interpretation of computed data.


N-Benzyl-2-[4-(trifluoromethyl)phenyl]acetamide (4a)
Yield: 65%; white solid; mp 134–136 °C.
1H NMR (600 MHz, CDCl3): δ = 7.61 (d, J = 8.05 Hz, 2 H), 7.41 (d, J = 8.00 Hz, 2 H), 7.32 (d, J = 7.53 Hz, 2 H), 7.28–7.34 (m overlapping d at 7.32 ppm, 1 H), 7.22 (d, J = 7.13 Hz, 2 H), 5.89 (br. s, 1 H), 4.43 (d, J = 5.8 Hz, 2 H), 3.64 (s, 2 H).
13C NMR (150.0 MHz, CDCl3): δ = 169.7, 138.8 (br s), 137.8, 129.6, 129.59 (q, J C–F = 32.5 Hz), 128.7, 127.62, 127.59, 125.8 (q, J C–F = 3.8 Hz), 124.0 (q, J C–F = 272.0 Hz), 43.8, 43.3.
IR (neat): 3238, 3063, 1625, 1556, 1328, 1122, 1070, 753, 698 cm–1.
HRMS: m/z [M + H]+ calcd for C15H15F3NO: 294.1100; found: 294.1090.
/////////////////
The continuous flow Barbier reaction: an improved environmental alternative to the Grignard reaction?
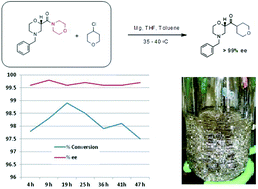
A key pharmaceutical intermediate (1) for production of edivoxetine·HCl was prepared in >99% ee via a continuous Barbier reaction, which improves the greenness of the process relative to a traditional Grignard batch process. The Barbier flow process was run optimally by Eli Lilly and Company in a series of continuous stirred tank reactors (CSTR) where residence times, solventcomposition, stoichiometry, and operations temperature were optimized to produce 12 g h−1crude ketone 6 with 98% ee and 88% in situ yield for 47 hours total flow time. Continuous salt formation and isolation of intermediate 1 from the ketone solution was demonstrated at 89% yield, >99% purity, and 22 g h−1 production rates using MSMPRs in series for 18 hours total flow time. Key benefits to this continuous approach include greater than 30% reduced process mass intensity and magnesium usage relative to a traditional batch process. In addition, the flow process imparts significant process safety benefits for Barbier/Grignard processes including >100× less excess magnesium to quench, >100× less diisobutylaluminum hydride to initiate, and in this system, maximum long-term scale is expected to be 50 L which replaces 4000–6000 L batch reactors.
A continuous flow Barbier reaction was employed for the production of a key pharmaceutical intermediate (1) in the synthesis of edivoxetine·HCl (a highly selective norepinephrine re-uptake inhibitor).
 US scientists from Eli Lilly and Company and D&M Continuous Solutions, led by Michael Kopach,
report the development of a continuous Barbier reaction which preserves
chirality and the product obtained in >99% ee. The team ran the
process in a series of continuous stirred tank reactors, where residence
time, solvent composition, stoichiometry and operations temperature
were optimised to produce 12 g per hour of the ketone precursor to 1 with 98% ee and 88% in situ yield for 47 hours total flow time. Continuous salt formation and isolation of 1 could then be achieved from the ketone solution with >99% purity.
US scientists from Eli Lilly and Company and D&M Continuous Solutions, led by Michael Kopach,
report the development of a continuous Barbier reaction which preserves
chirality and the product obtained in >99% ee. The team ran the
process in a series of continuous stirred tank reactors, where residence
time, solvent composition, stoichiometry and operations temperature
were optimised to produce 12 g per hour of the ketone precursor to 1 with 98% ee and 88% in situ yield for 47 hours total flow time. Continuous salt formation and isolation of 1 could then be achieved from the ketone solution with >99% purity.This process offers up several significant advantages over a traditional Grignard batch process. This continuous flow method gave greater than 30% reduced process mass intensity and magnesium usage relative to the batch method. Equally, the flow process resulted in >100 x less excess magnesium to quench and >100 x less diisobutylaluminum hydride to initiate giving significant safety benefits. The authors expect that the maximum long-term scale of the process is 50 L which would replace 4000-6000 L batch reactors.
Continuous Flow Barbier Reaction

Figure 2. Continuous Barbier Laboratory Setup
For
100 years, Grignard reactions have been one of the most powerful and
effi cient organic chemistry methodologies for C-C bond formation.
However, Grignard reactions are also among the most challenging
reactions from both operational and potential safety issues due to
initiation diffi culties and runaway potential. A close variation to the
Grignard reaction is the Barbier reaction wherein the Grignard reagent
is prepared in the presence of an electrophile resulting in the
immediate consumption of the Grignard. A Barbier reaction using a CSTR
was developed for a key pharmaceutical intermediate in production of
edivoxetine·HCl (Scheme 4) [9]. In the fl ow setup (Figure 2),
solid magnesium is sequestered in the fi rst tank where the Grignard
initiation event takes place. CSTR 2 was used as an aging tank and CSTR 3
was the quench tank. CSTRs were used for Grignard reaction rather than a
PFDR because of the solid Mg reagent.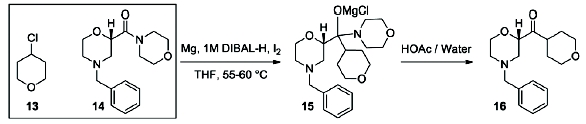
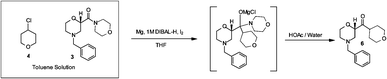
Scheme 4: Barbier Reaction to form Ketone 15
Continuous
reaction improved process safety, product quality, and process
greenness. The continuous reaction achieved >99% ee in situ versus
95% ee batch because of immediate conversion of unstable intermediate.
Solvent volumes were reduced 30%. The safety hazards were reduced by
decreasing the reactor size by 50X, which reduced chemical potential and
also increased heat transfer surface area per unit volume by 4X.
DIBAL-H initiating agent was reduced by more than 100X, and excess Mg
that must be quenched at the end of reaction was almost eliminated. When
run continuously, the commercial scale Grignard formation reactor was
expected to be 50L, which replaces 4000-6000L batch reactor.The continuous flow Barbier reaction: an improved environmental alternative to the Grignard reaction?
Michael E. Kopach,*a Dilwyn J. Roberts,a Martin D. Johnson,a Jennifer McClary Groh,a Jonathan J. Adler,b John P. Schafer,b Michael E. Kobierskia and William G. Tranklea
*Corresponding authors
aChemical Product Research and Development, Eli Lilly and Company, Indianapolis, USA
E-mail: kopach_michael@lilly.com
E-mail: kopach_michael@lilly.com
bD&M Continuous Solutions, Indianapolis, USA
Green Chem., 2012,14, 1524-1536
DOI: 10.1039/C2GC35050E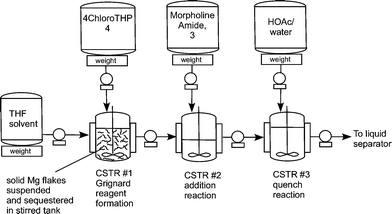
Three vessel Grignard CSTR process train.
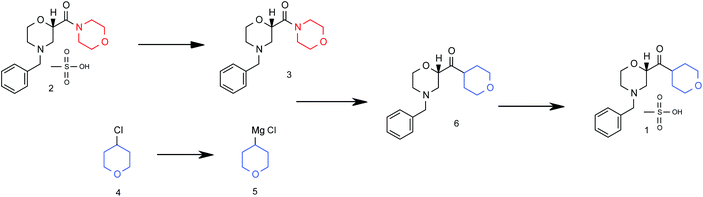
Grignard synthesis of compound 1.
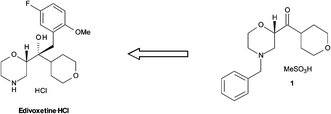
Retrosynthesis of edivoxetine·HCl.
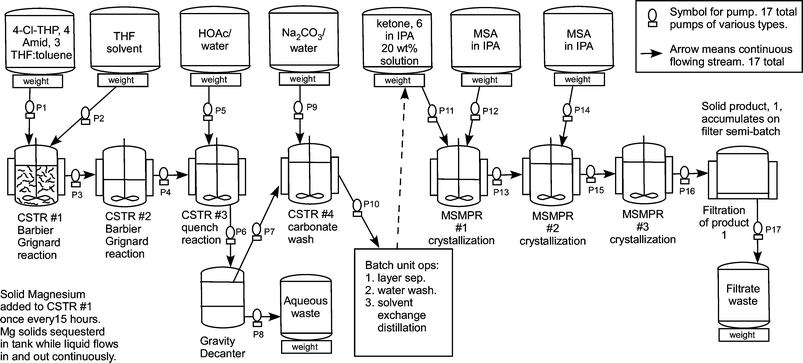 Flow diagram for the whole continuous process from amide 3 to product 1.
Flow diagram for the whole continuous process from amide 3 to product 1.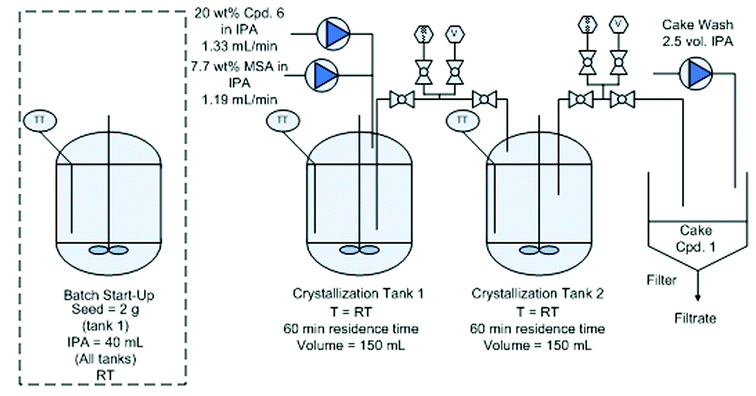
Continuous crystallization of compound 1.

Distillation and continuous crystallization of compound 1.
Entry, Rxn temp. (°C), Vol. ratio THF–toluene (%), Conversion (%), ee (%)
//////////The continuous flow, Barbier reaction, improved environmental alternative, Grignard reaction, FLOW SYNTHESIS

Friday, 29 July 2016
HPLC-free Protein Synthesis
HPLC-free Protein Synthesis
First methode for the fast chemical total synthesis of proteins without HPLC-purification
Read more
Water and n-Butyllithium Together At Last
Water and n-Butyllithium
Together At Last
Cycloaddition of nitrone ylides with aldehydes using catalytic amounts of nBuLi
Read more
Improved Catalyst for Hydrogen Production
Improved Catalyst
for Hydrogen Production
Engineering water dissociation sites by doping molybdenum disulfide
Read more
A Milder Way to SCF3 Incorporation
A Milder Way to SCF3
Incorporation
Perfluoroalkylthiolation of alkynes under mild, base-free conditions
Read more
Subscribe to:
Comments (Atom)