Fatty
acid sugar esters are non-toxic, odorless, non-irritanting surfactants.
They can be synthesized by renewable resources and are completely
biodegradable in aerobic and anaerobic conditions. Their application has
been expanded in innumerous areas including pharmaceuticals, cosmetics,
detergents and food industry. Lipase-catalyzed esterification have been
investigated as a potential substitute to the traditional chemical,
demanding milder reaction conditions, allowing better reaction control
and providing higher-quality products. So, the lipase catalyzed sugar
ester synthesis becomes an interesting strategy for producing
biodegradable, non- ionic surfactants. The main disadvantage of this
protocol is the poor solubility of substrates and long reaction time
required for performed the esterification reaction with moderated to
good yields.
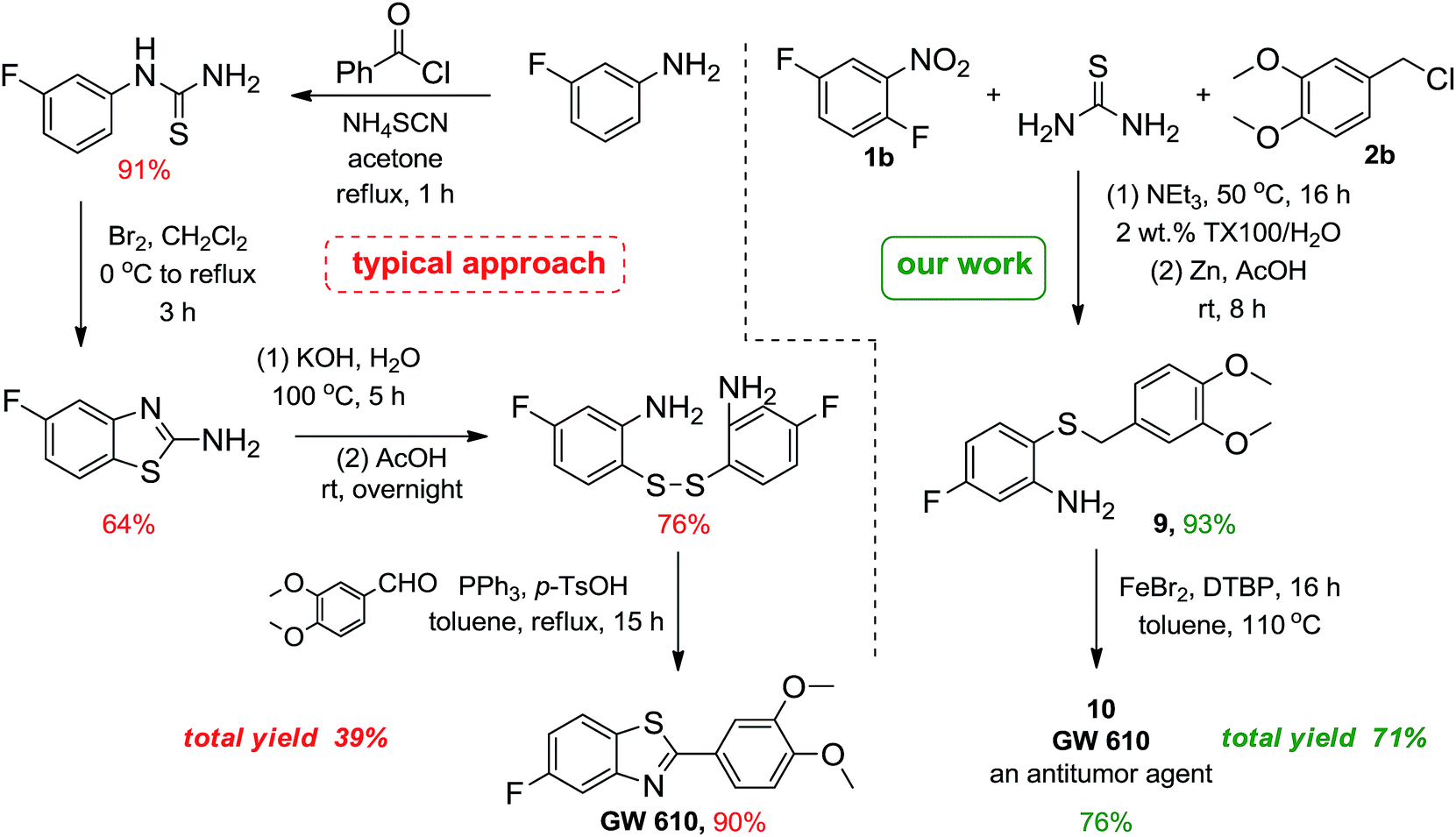
Synthesis of 2,3:4,5-O-diisopropylidene-D-frutopyranose (FK) (2)
In
a 2000 mL reactor was added 30 g (44.8 mmol) of sucrose and 400 mL of
acetone being vigorously mechanically stirred at 5°C for 15 min. Then,
16 mL of concentrate sulfuric acid (H
2SO
4) was
slowly added to the reaction mixture. The solution was kept under
stirring for 150 min. Subsequently, the reaction mixture was cooled
(0–10°C) in ice bath and neutralized with 50% NaOH (w/v). The pH was
adjusted with saturated sodium carbonate. The final mixture was filtered
to remove the solids and subsequently, the solvent was evaporated under
reduced pressure. The solid crude ketal was diluted with 400 mL of
dichloromethane. A 0.5 M H
2SO
4 solution was added
and stirred vigorously for 120 min. The organic phase was separated and
washed consecutively with sodium bicarbonate (NaHCO
3) and water and dried with anhydrous sodium sulfate (Na
2SO
4).
The solvent was evaporated under reduced pressure until obtaining a
white solid, which was crystallized in hexane with 30% final yield after
filtration through activated charcoal
[
19].
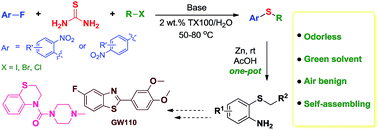
Continuous flow reaction procedure
An equimolar stock solution (
tert-butylmethyl ether (MTBE), toluene or
p-cymene) of 2,3:4,5-
O-D-diisopropylidene
frutopyranose (FK) and the RePO was prepared (the molarity of the
residue was expressed in palmitic acid). The starting mixture was
stirred for 5 min while the instrument Asia Flow Reactor was equipped
with Omnifit column (2.4 mL) containing the immobilized lipase from
R. miehei(600 mg).
The reaction parameters were selected on the flow reactor, and
processing was started, whereby only pure solvent was pumped through the
system until the instrument had achieved the desired reaction
parameters and stable processing was assured. At this point, the inlet
pipe of the flask was switched to HPLC bottle containing the prepared
reaction mixture. After processing through the flow reactor, the inlet
tube was dipped back into the flask containing respective pure solvent
and processed in order to wash the system of any remaining reactant.
Enhanced production of fructose ester by biocatalyzed continuous flow process
Felipe K Sutili1,
Halliny S Ruela1,
Daniel De O Nogueira12,
Ivana CR Leal2,
Leandro SM Miranda1 and
Rodrigo OMA De Souza1*
1Biocatalysis
and Organic Synthesis Group, Chemistry Institute, Federal University of
Rio de Janeiro, Rio de Janeiro CEP 22941 909, Brazil
2Faculdade de Farmácia, Federal University of Rio de Janeiro, Rio de Janeiro CEP22941909, Brazil
http://www.sustainablechemicalprocesses.com/content/3/1/6
Sustainable Chemical Processes 2015,
3:6
doi:10.1186/s40508-015-0031-8
The electronic version of this article is the complete one and can be found online at:
http://www.sustainablechemicalprocesses.com/content/3/1/6

-
Departamento de Química Orgânica
Rio de Janeiro, RJ, Brazil
https://www.researchgate.net/profile/Rodrigo_De_Souza4
/////