Green Chem., 2018, Advance Article
DOI: 10.1039/C7GC03425C, Paper
DOI: 10.1039/C7GC03425C, Paper
Weigang Fan, Yves Queneau, Florence Popowycz
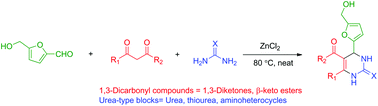
The use of the renewable platform molecule 5-hydroxymethylfurfural (HMF) in the multi-component Biginelli reaction has been investigated.
The use of the renewable platform molecule 5-hydroxymethylfurfural (HMF) in the multi-component Biginelli reaction has been investigated.
HMF in multicomponent reactions: utilization of 5-hydroxymethylfurfural (HMF) in the Biginelli reaction
Abstract
The use of the renewable platform molecule 5-hydroxymethylfurfural (HMF) in the multi-component Biginelli reaction has been investigated. Multicomponent reactions (MCR) using HMF offer straightforward access to novel fine chemicals. However, the peculiar reactivity and lower stability of HMF have limited its use in such strategies. In this paper, we report our results on the use of HMF in 3-component Biginelli reactions, leading in one single step to a series of functionalized dihydropyrimidinones obtained in moderate to good yields, with a broad substrate scope of 1,3-dicarbonyl compounds and urea building blocks. This is the first report on the use of HMF in this reaction. The CH2OH motif found in HMF provides useful functionalization for the target molecules, which cannot be offered by simpler aldehydes such as furfural.
5-Acetyl-4-[5’-(hydroxymethyl)furan-2’-yl]-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4a):
5-Acetyl-4-[5’-(hydroxymethyl)furan-2’-yl]-6-methyl-3,4-dihydropyrimidin-2(1H)-one (4a):
Reaction time: 8 h; Global yield: 86%; (78% yield after simple filtration + additional 8% yield after purification of the filtrate by column chromatography).
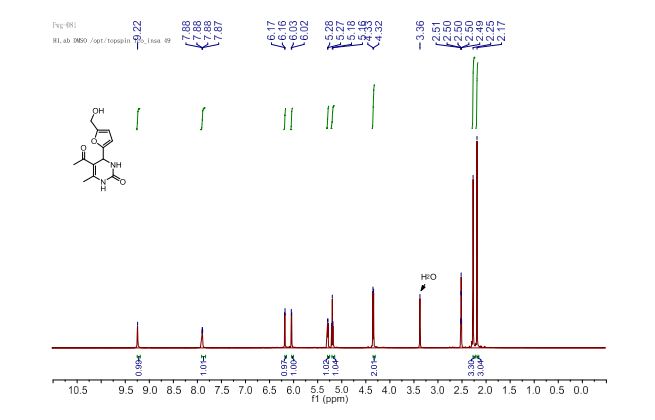
1H NMR (400 MHz, DMSO-d6) δ 9.22 (d, 1H, J = 1.2 Hz, H1), 7.88 (dd, 1H, J = 3.4, 1.2 Hz, H3), 6.16 (d, 1H, J = 3.1 Hz, H4’), 6.03 (d, 1H, J = 3.1 Hz, H3’), 5.27 (d, 1H, J = 3.4 Hz, H4), 5.18 (t, 1H, J = 5.6 Hz, OH), 4.33 (d, 2H, J = 5.6 Hz, CH2), 2.25 (s, 3H, CH3-C6), 2.17 (s, 3H, CH3CO).
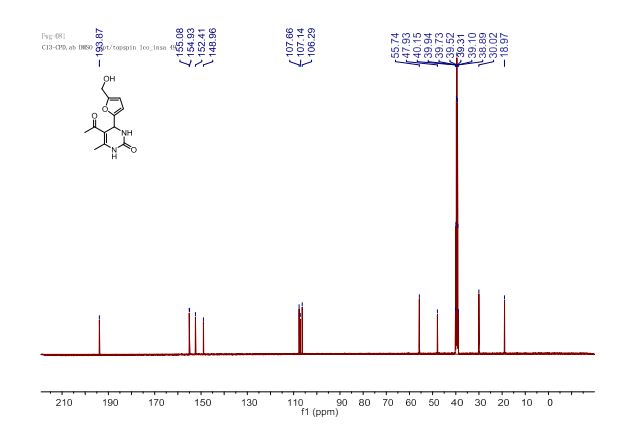
13C NMR (100 MHz, DMSO-d6) δ 193.9 (COCH3), 155.1, 154.9 (C2’, C5’), 152.4 (C2), 149.0 (C6), 107.7 (C4’), 107.1 (C5), 106.3 (C3’), 55.7 (CH2OH), 47.9 (C4), 30.0 (CH3CO), 19.0 (CH3-C6).
HRMS (ESI) m/z: Calcd for [M+Na]+ C12H14N2NaO4 273.0846; Found 273.0850.

1H NMR (400 MHz, DMSO-d6) δ 9.22 (d, 1H, J = 1.2 Hz, H1), 7.88 (dd, 1H, J = 3.4, 1.2 Hz, H3), 6.16 (d, 1H, J = 3.1 Hz, H4’), 6.03 (d, 1H, J = 3.1 Hz, H3’), 5.27 (d, 1H, J = 3.4 Hz, H4), 5.18 (t, 1H, J = 5.6 Hz, OH), 4.33 (d, 2H, J = 5.6 Hz, CH2), 2.25 (s, 3H, CH3-C6), 2.17 (s, 3H, CH3CO).
13C NMR (100 MHz, DMSO-d6) δ 193.9 (COCH3), 155.1, 154.9 (C2’, C5’), 152.4 (C2), 149.0 (C6), 107.7 (C4’), 107.1 (C5), 106.3 (C3’), 55.7 (CH2OH), 47.9 (C4), 30.0 (CH3CO), 19.0 (CH3-C6).
HRMS (ESI) m/z: Calcd for [M+Na]+ C12H14N2NaO4 273.0846; Found 273.0850.
Research experience

Université de Lyon, INSA Lyon, ICBMS, Equipe Chimie Organique et Bioorganique, UMR 5246 CNRS, Université Lyon 1, CPE Lyon, Bâtiment Jules Verne, 20 Avenue Albert Einstein, F-69621 Villeurbanne Cedex, France
E-mail: florence.popowycz@insa-lyon.fr
E-mail: florence.popowycz@insa-lyon.fr

Yves QUENEAU
CNRS Research Director chez ICBMS INSA Lyon Univ Lyon - Carbohydrate Chemistry
ICBMS INSA Lyon University of Lyon

Université de Lyon, INSA Lyon, ICBMS, Equipe Chimie Organique et Bioorganique, UMR 5246 CNRS, Université Lyon 1, CPE Lyon, Bâtiment Jules Verne, 20 Avenue Albert Einstein, F-69621 Villeurbanne Cedex, France
E-mail: yves.queneau@insa-lyon.fr,
E-mail: yves.queneau@insa-lyon.fr,

 .
. Corresponding author email mh_1395@yahoo.com
Corresponding author email mh_1395@yahoo.com