Synthesis of tetrazines from gem-difluoroalkenes under aerobic conditions at room temperature
An efficient and green procedure for the synthesis of tetrazines has been developed based on an old chemistry reported by Carboni in 1958. Both symmetric and asymmetric 3,6-disubstituted 1,2,4,5-tetrazines can be obtained in moderate to high yields from the corresponding gem-difluoroalkenes under aerobic conditions at room temperature. This work represents a rare example that ambient air is utilized as an oxidant for the synthesis of tetrazines.
Synthesis of tetrazines from gem-difluoroalkenes under aerobic conditions at room temperature
*
Corresponding authors
a
National Engineering Research Center for Carbohydrate Synthesis, Jiangxi Normal University, Nanchang 330022, P. R. China
E-mail: huxiangg@iccas.ac.cn
E-mail: huxiangg@iccas.ac.cn
b
Beijing National Laboratory for Molecular Science (BNLMS), CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
Green Chem., 2017, Advance Article
DOI: 10.1039/C6GC03494B
http://pubs.rsc.org/en/Content/ArticleLanding/2017/GC/C6GC03494B?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
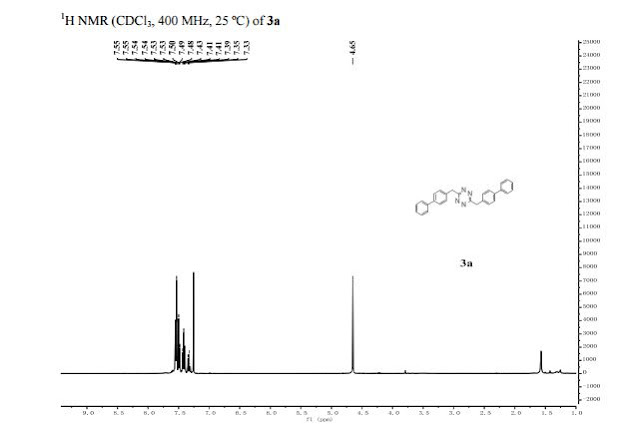
3,6−bis([1,1'−biphenyl]−4−ylmethyl)−1,2,4,5−tetra zine (3a). (41 mg, 83%). purple solid;
m.p. 200−202°C;
IR(KBr) nmax/cm−1 2924, 2850, 1488, 1451, 1432, 1388, 851, 750;
1 H NMR (400 MHz, CDCl3) 7.55−7.33 (m, 18H), 4.65 (s, 4H).
13C NMR (100 MHz, CDCl3) δ 169.2, 140.6, 140.4, 134.8, 129.7, 128.8, 127.6, 127.4, 127.1, 40.9;
HRMS (ESI): calcd. for C28H22N4 [M+H]+ 415.19172, found 415.19124.
///////


No comments:
Post a Comment