Chemical Journal of Chinese Universities
DOI: 10.3969/j.issn.0251-0790.2012.09.016 http://www.cjcu.jlu.edu.cn/EN/abstract/abstract24471.shtml# http://www.cjcu.jlu.edu.cn/EN/volumn/next.shtml
-
Chemical Journal of Chinese Universities About Journal
www.cjcu.jlu.edu.cn/EN/column/column104.shtml
“Chemical Journal of Chinese Universities”(Chinese Edition) is a comprehensive academic journal in the field of chemistry sponsored by Jilin University and ...
| YU Hai-Feng1, LIAO Pei-Qiu2 |
| 1. School of Chemistry and Life Science, Anshan Normal University, Anshan 114007, China; 2. Department of Chemistry, Northeast Normal University, Changchun 130024, China |
|
|||
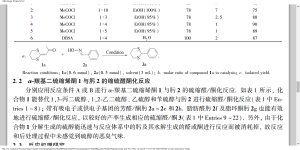
| Abstract Thioacetals are important compounds because they can be considered as both useful protecting groups of carbonyl compounds in the synthesis of multi-functional complex molecules and acyl carbanion equi-valents in C—C bond forming reactions. Since many reactions have been developed to prepare oximes from non carbonyl compounds, to lead to a novel and efficient method for thioacetal preparation, transthioacetalization of oximes has received more and more attention. Unfortunately, the transformation usually suffers from the use of harmful, odorous thiols which can lead to serious safety and environment problems. From the green chemistry point of view, an efficient and odorless transthioacetalization of oximes involving an environment friendly reagent is of great importance and necessity. In this work, using odorless and stable α-oxo ketene dithioacetals 1 as thiol equivalents, the thioacetalization reaction of oximes 2 were studied. In the reaction system of MeCOCl-EtOH(95%) or 4-dodecylbenzenesulfonic acid(DBSA)-H2O, the thioacetalization reaction were carried out in reflux temperature. It is noteworthy that the odor of thiols can not be perceived during either the reaction or workup. | |||
| Odorless and Efficient Thioacetalization Reaction of Oximes[J]. Chemical Journal of Chinese Universities, 2012, 33(09): 1969-1972. |
| URL: |
| http://www.cjcu.jlu.edu.cn/EN/10.3969/j.issn.0251-0790.2012.09.016 OR http://www.cjcu.jlu.edu.cn/EN/Y2012/V33/I09/1969 |

Anshan Normal University, Anshan 114007, China
Department of Chemistry, Northeast Normal University, Changchun 130024, China - See more at: http://organicsynthesisinternational.blogspot.in/#sthash.dl5jq4SB.dpuf
.png)
.png)
.png)



