| Synthesis of 5-amino-3-t-butyl-1-(2', 4'-dinitro)phenyl-1Hpyrazole (3) | |||||||||||||||||||||
| A mixture of 4, 4-dimethyl-3-oxo-pentanenitrile (1, 34 mM), 2, 4-dinitrophenyl hydrazine hydrochloride (2, 35 mM) and 50 mL absolute ethanol along with few drops of AcOH were heated at the reflux temperature for overnight and cooled to room temperature. The mixture was evaporated under vacuum and the residue thus obtained was washed with ether, suspended in EtOAc, and treated with 1 M NaOH solution. The organic layer then separated, washed with brine, dried over anhydrous magnesium sulphate and concentrated. The solid which separated was collected, then washed with a mixture of ether and hexane to give 5-amino-3-t-butyl-1-(2', 4'-dinitro)phenyl-1H-pyrazole (3) [22]. | |||||||||||||||||||||
| General procedure for N-[3'-t-butyl-1'-(2", 4"-dinitro) phenylpyrazol-5'-yl] benzamide (5a-l) | |||||||||||||||||||||
| To a solution of 5-amino-3-t-butyl-1-(2', 4'-dinitro)phenyl-1Hpyrazole (3, 9.3 mM) in dichloromethane (5 mL), triethyl amine (23 mM) was added drop wise. The appropriate benzoylchlorides (4a-k, 12 mM) in dichloromethane were added to the above reaction mixture drop wise and stirred for 3 hours at room temperature. The mixture was further diluted with dichloromethane and washed with water, brine followed by once again with water. Sodium sulfate was used to dry the organic layer; solvent was removed under vacuum to get the final derivatives (5a-l). All the compounds were purified over silica to get pure N-(3-tert-butyl-2, 4-dinitro-1-phenyl-1H-pyrazol-5-yl) benzamides (5a-l). | |||||||||||||||||||||
Compound characterization | |||||||||||||||||||||
| N-[3'-t-butyl-1'-(2", 4"-dinitro)phenylpyrazol-5'-yl] benzamide 5a: Yield: 85%, m.p.: 190-192°C, IR (KBr) ν cm-1: 3362 cm-1 (NH str.), 3020 cm-1 (CH str. aromatic), 2950 cm-1 (CH str. methyl), 1643 cm-1 (NH bnd) 1H NMR (CDCl3) δ (ppm): 1.23 (s, 9H, t-butyl), 6.18 (s, 1H, C4, pyrazole), 7.15-7.55 (m, 3H, C3', C4', C5' aromatic), 7.7-7.8 (m, 2H, C5, C6, aromatic), 7.9-8.1 (d, 2H, C2', C6', aromatic), 8.2 (s, 1H, C3, aromatic), 9.61 (bs, 1H, NH amide). ESI-MS (m/z): 410, 100% [M+H]+.
| |||||||||||||||||||||
Organic Synthesis International by Dr Anthony Melvin Crasto Ph.D, Worlddrugtracker, Million hits on google on all sites, One lakh connections worldwide. Pushing boundaries.Interaction site for Organic chemists worldwide, Mail me at amcrasto@gmail.com if you like me
Pages
- Home
- THESIS
- orgsyn.org site
- Chemspider synthetic pages
- ABOUT ME
- DISCLAIMER
- Montelukast and similar drugs
- Glitazar and glitazone series
- ABAN SERIES
- melteon series
- USEFUL LINKS
- LITERATURE SEARCH
- DIPINE SERIES
- International spaces
- GLIPTIN SERIES 2/2
- Atom Economy (AE), Reaction Mass Efficiency (RME) ...
Thursday, 14 January 2016
Design, Synthesis, Antimicrobial and Anti-inflammatory Activity of N-Pyrazolyl Benzamide Derivatives
Sunday, 10 January 2016
Cyclopropanes in water: a diastereoselective synthesis of substituted 2H-chromen-2-one and quinolin-2(1H)-one linked cyclopropanes
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02443A, Paper
DOI: 10.1039/C5GC02443A, Paper
Ashish Anand, Jayashree Yenagi, J. Tonannavar, Manohar V. Kulkarni
A one-pot three component reaction has been developed for the synthesis of substituted cyclopropanes employing 4-bromomethyl-2H-chromen-2-one/quinolin-2(1H)-ones, aromatic aldehydes and activated nitriles.
A one-pot three component reaction has been developed for the synthesis of substituted cyclopropanes employing 4-bromomethyl-2H-chromen-2-one/quinolin-2(1H)-ones, aromatic aldehydes and activated nitriles.
A one-pot three component reaction has been developed for the synthesis of substituted cyclopropanes employing 4-bromomethyl-2H-chromen-2-one/quinolin-2(1H)-ones, aromatic aldehydes and activated nitriles. The room temperature reaction in aqueous medium has been found to be diastereoselective and high yielding.
http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C5GC02443A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract
2-(6-Methyl-2-oxo-2H-chromen-4-yl)-3-phenyl-cyclopropane-1,1-dicarbonitrile (4a)
4a, (R= -H, R’= 6-CH3, R”= -CN, X=O)
For a typical compound 4a, (R= -H, R’= 6-CH3, R”= -CN, X=O) IR spectrum showed band at 2246 cm-1 (cyanide) and 1720 cm-1 (lactone). Further, the formation of cyclopropane ring was confirmed by the 1H-NMR wherein the C5-H of coumarin ring resonated at 7.83 ppm as a doublet with 5JC5-H = 1.2 Hz showing a 5-bond coupling with the C4-CH of coumarin ring. C4- CH of coumarin ring appeared at 4.83 ppm as doublet of doublets with Jvic(CH) = 8.8 Hz, Cou-CH 5JC5-H = 1.2 Hz. CH attached to aryl group resonated at 4.61 ppm as a doublet with Jvic(CH) = 8.8 Hz. C6-CH3 protons appeared at 2.45 as a singlet. C3-H was observed as a singlet at 6.82 ppm. Other aromatic protons resonated between 7.68-7.41 ppm. In 13C-NMR, the carbon attached to two cyano group resonated at 15.08 ppm, the methyl carbon appeared at 20.55 ppm. Cyclopropane ring carbon attached to aryl group resonated at 32.81 ppm whereas the one attached to coumarin appeared at 36.78 ppm. Carbon of two cyano group resonated at 112.68 and 112.91 ppm. The lactone carbon of the coumarin ring appeared at 159.35 ppm. Aromatic carbons resonated in the range of 112-159 ppm. Formation of the product was further confirmed by EI-MS where the molecular ion peak was observed at 326 m/z
2-(6-Methyl-2-oxo-2H-chromen-4-yl)-3-phenyl-cyclopropane-1,1-dicarbonitrile (4a) White; Yield 85%;
m.p: 268-270°C;
IR (KBr) cm-1 1720 (C=O lactone), 2246 (CN);
1H-NMR (400 MHz, DMSO-d6, TMS) δ (ppm): 7.83(d, 1H, 5JC5-H = 1.2 Hz, C5-H), 7.68-7.41(m, 7H, ArH), 6.82(s, 1H, C3-H), 4.83(dd, 1H, Jvic(CH) = 8.8Hz, Cou-CH, 5JC5-H = 1.2 Hz), 4.61(d, 1H, Jvic(CH) = 8.8Hz, Ar-CH), 2.45(s, 3H, -CH3);
13C-NMR (100 MHz, DMSO-d6) δ (ppm): 8.57, 15.08, 20.55, 32.81, 36.78, 45.73, 112.68, 112.91, 115.95, 116.69, 117.64, 124.60, 128.62, 129.00,129.12, 130.90, 133.63, 134.23, 146.64, 151.12, 159.35;
MS m/z 326(100%);
Anal Calcd. for C21H14N2O2 (%), Calcd: C, 77.29; H, 4.32; N, 8.58; found: C, 77.26; H, 4.29; N, 8.55
Cyclopropanes in water: a diastereoselective synthesis of substituted 2H-chromen-2-one and quinolin-2(1H)-one linked cyclopropanes
Cyclopropanes in water: a diastereoselective synthesis of substituted 2H-chromen-2-one and quinolin-2(1H)-one linked cyclopropanes
*
Corresponding authors
a
Department of Studies in Chemistry, Karnatak University, Pavate Nagar, Dharwad 580003, India
E-mail: manohar274@gmail.com
E-mail: manohar274@gmail.com
b
Department of Studies in Physics, Karnatak University, Pavate Nagar, Dharwad 580003, India
Green Chem., 2016, Advance Article
DOI: 10.1039/C5GC02443A http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C5GC02443A?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+rss%2FGC+%28RSC+-+Green+Chem.+latest+articles%29#!divAbstract http://www.rsc.org/suppdata/c5/gc/c5gc02443a/c5gc02443a1.pdf ///////////////////////// c1(ccc4c(c1C2[C@@H](C2(C#N)C#N)c3ccccc3)\C=C/C(O4)=O)C
Sunday, 3 January 2016
Palladium(II) porphyrin – anthracene dyad bridged via short and conformationally rigid bicyclo[2.2.2]octadiene spacer
The synthesis and photophysical characterization of a palladium(II) porphyrin – anthracene dyad bridged via short and conformationally rigid bicyclo[2.2.2]octadiene spacer were achieved. A spectroscopic investigation of the prepared molecule in solution has been undertaken to study electronic energy transfer in excited singlet and triplet states between the anthracene and porphyrin units. By using steady-state and time-resolved photoluminescence spectroscopy it was shown that excitation of the singlet excited state of the anthracene leads to energy transfer to the lower-lying singlet state of porphyrin. Alternatively, excitation of the porphyrin followed by intersystem crossing to the triplet state leads to very fast energy transfer to the triplet state of anthracene. The rate of this energy transfer has been determined by transient absorption spectroscopy. Comparative studies of the dynamics of triplet excited states of the dyad and reference palladium octaethylporphyrin (PdOEP) have been performed.
SEE..................
http://pubs.rsc.org/en/content/articlelanding/2015/dt/c5dt03784k#!divAbstract
Paper
Interplay between singlet and triplet excited states in a conformationally locked donor–acceptor dyad
Mikhail A. Filatov,*ab
Fabian Etzold,a
Dominik Gehrig,a
Frédéric Laquai,ac
Dmitri Busko,a
Katharina Landfestera and
Stanislav Baluschevade
*
Corresponding authors
a
Max Planck
Institute for Polymer Research, Ackermannweg 10, D-55128 Mainz, Germany
E-mail: filatovm@tcd.ie
E-mail: filatovm@tcd.ie
b
Institute of
Polymers, Bulgarian Academy of Sciences, Acad. G. Bonchev Str., block
103-A, BG – 1113 Sofia, Bulgaria
c
Physical Sciences
and Engineering Division (PSE), Material Science and Engineering (MSE),
Solar and Photovoltaics Engineering Research Center (SPERC), King
Abdullah University of Science and Technology (KAUST), Thuwal
23955-6900, Kingdom of Saudi Arabia
d
Optics and
Spectroscopy Department, Faculty of Physics, Sofia University “St.
Kliment Ochridski”, 5 James Bourchier, 1164 Sofia, Bulgaria
e
Freiburg Institute
for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg,
Albertstraße 19, D-79104 Freiburg, Germany
Dalton Trans., 2015,44, 19207-19217
DOI: 10.1039/C5DT03784K

Mikhail A Filatov
Dr.
Marie Curie Postdoctoral Research Fellow
Trinity College Dublin, Dublin · School of Chemistry
Research Experience
- Sep 2015–
presentMarie Curie Postdoctoral Research Fellow
Trinity College Dublin · School of ChemistryIreland · Dublin - Mar 2014–
Sep 2015Research Associate
Institute of Polymers, Bulgarian Academy of SciencesBulgaria · Sofia, Sofia-Capital - Feb 2010–
Mar 2014Postdoctoral researcher
Max Planck Insitute for Polymer ResearchGermany · Mainz - Dec 2008–
Dec 2009Postdoctoral researcher
Institut de Chimie Moléculaire de l'Université de Bourgogne, CNRSFrance · Dijon
Education
- Sep 2000–
Oct 2008Lomonosov Moscow State University
Chemistry · MS and PhDRussia · Moscow
Awards & achievements
- Feb 2015Award: Marie Skłodowska-Curie Individual Fellowship
- Oct 2007Scholarship: • Scholarship of the President of Russian Federation for outstanding PhD students
- Sep 2005Grant: • Grant from the Russian Foundation for Assistance to Small Innovative Enterprises (FASIE) for establishing a start-up company with the innovative project “Development of Technology of 24-Epibrassinolide Production”
Other
- LanguagesBulgarian, English, German, Russian
- Journal
RefereeThe Journal of Organic Chemistry - Other InterestsReading, hiking, diving, traveling
/////////
Saturday, 2 January 2016
Fluoxastrobin
Fluoxastrobin

Fluoxastrobin
Fluoxastrobin; Disarm; Fluoxastrobin [ISO]; UNII-XQ43WY091Y; HEC 480 SC;
(E)-1-[2-[6-(2-chlorophenoxy)-5-fluoropyrimidin-4-yl]oxyphenyl]-1-(5,6-dihydro-1,4,2-dioxazin-3-yl)-N-methoxymethanimine
- (1E)-(2-((6-(2-chlorophenoxy)-5-fluoro-4-pyrimidinyl)oxy)phenyl)(5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyloxime
- 361377-29-9
- 193740-76-0
| Molecular Formula: | C21H16ClFN4O5 |
|---|---|
| Molecular Weight: | 458.826943 g/mol |
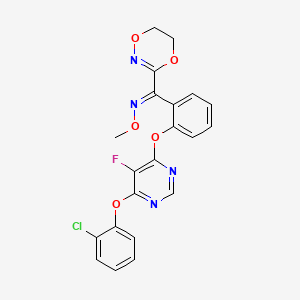
103-108 deg C
MacBean
C, ed; e-Pesticide Manual. 15th ed., ver. 5.1, Alton, UK; British Crop
Protection Council. Fluoxastrobin (361377-29-9) (2008-2010)
http://fluoridealert.org/wp-content/pesticides/fluoxastrobin.2004.article.pdf
http://fluoridealert.org/wp-content/pesticides/fluoxastrobin.2004.article.pdf
PAPER
http://pubs.rsc.org/en/content/articlehtml/2015/gc/c5gc00402k
PATENT
US-9193698-B2 / 2015-11-24
Process for preparing fluoxastrobin
(E)-(2-((6-(2-chlorophenoxy)-5-fluoropyrimidin-4-yl)oxy)phenyl)(5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyl oxime [Fluoxastrobin]
To a solution of (E)-(2-((6-chloro-5-fluoropyrimidin-4-yl)oxy)phenyl)(5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyl oxime (14)(100 g, 0.564 mol) in toluene was added 2-chlorophenol (54 g, 0.846 mol), K2CO3 (50
g, 0.733 mol), and DMF (50 mL) at ambient temperature. The reaction
mixture was stirred at 50-60° C. for 3-4 h. The progress of the reaction
was monitored by the HPLC analysis. Upon completion of the reaction,
aqueous NaOH (10%) (200 mL) was charged followed by water (300 mL). The
mixture was stirred and the toluene layer was separated. The toluene layer was washed with a solution of brine (600 mL). The final toluene layer was recovered completely to get the crude product. To the above crude product, methanol
was charged and heated to 60° C. until the clear solution is formed.
The solution was stirred at room temperature to get the pure product
precipitated. The pure fluoxastrobin product was filtered and washed with methanol. The product was further dried to obtain the pure fluoxastrobin product meeting the desired specifications. Yield—75-88%.
IR (cm−1,
KBr) 3072.99w, 2981.58w, 2936.76s, 2819.79w, 2502.01w, 1601.14s,
1572.37s, 1447.88s, 1305.43m, 1268.11m, 1217.15m, 1191.21m, 1092.60m,
1049.05m, 1001.26w, 910.25w, 762.81w.
1H NMR (CDCl3,
400 MHz) δ 3.846 (s, 3H), 4.170-4.160 (t, J=4 Hz, 2H), 4.464-4.484 (t,
J=4 Hz, 2H), 7.261-7.295 (m, 2H), 7.322-7.409 (2, 4H), 8.069 (s, 1H).
13C NMR (CDCl3,
400 MHz) δ 63.103, 64.153, 64.550, 122.659, 123.259, 123.823, 125.712,
127.150, 127.397, 128.094, 130.511, 130.679, 130.776, 131.473, 134.138,
146.004, 148.166, 148.943, 150.354, 150.478, 151.819, 157.395, 157.466,
157.783, 157.854.
MS (EI) m/z 459.1 (M+1); MS2 (EI) m/z 427.1, 383.0,
366.9, 342.1, 306.2, 246.0, 231.1, 188.0.
HPLC (Area %): 99.40%. M.P.
108-112° C.
Isomerisation of (Z)-Fluoxastrobin to (E)-Fluoxastrobin using methane sulphonic acid. To a stirred solution of (Z)-Fluoxastrobin (0.3 g; 0.65 mmole) in acetonitrile (3 ml) was dropwise added methane sulphonic acid
(0.04 ml, 0.65 mmole) at an ambient temperature. The reaction mixture
was stirred for 2-3 hr at the same temperature. The progress of reaction
was monitored by thin layer chromatography (TLC). Dichloromethane
(5 ml) and DM water (5 ml) was added to reaction mass at an ambient
temperature. After vigorous stirring, the layers were separated. The
aqueous layer was back extracted with dichloromethane (5 ml) and the combined dichloromethane layer was washed with 10% aqueous sodium bicarbonate solution (20 ml) followed by washing with 10% brine solution (20 ml). Dichloromethane
was distilled off at reduced pressure at 35-45° C. to obtain
(E)-Fluoxastrobin as crude product (0.25 g, 83% of theoretical yield).
Crude fluoxastrobin on purification in ethanol affords pure (E)-Fluoxastrobin. Isolated product HPLC purity (% area): (Z)-fluoxastrobin: 1.02% and (E)-fluoxastrobin: 95.92%.
Isomerisation of (Z)-Fluoxastrobin to (E)-Fluoxastrobin using phosphoric acid. To a stirred solution of (Z)-Fluoxastrobin (0.25 g; 0.54 mmole) in acetonitrile (4 ml) was dropwise added phosphoric acid
(0.03 g, 0.54 mmole) at an ambient temperature. The reaction mixture
was stirred for 2-3 hr at the same temperature. Progress of reaction was
monitored by thin layer chromatography/HPLC. Dichloromethane
(5 ml) and DM water (5 ml) was added to reaction mass at an ambient
temperature. After vigorous stirring, layers were separated. The aqueous
layer was back extracted with dichloromethane (5 ml). The combined dichloromethane layers were washed with 10% aq. Sodium bicarbonate solution (20 ml) followed by washing with 10% brine solution (20 ml). Dichloromethane
was distilled off at reduced pressure at 40-45° C. to obtained
(E)-Fluoxastrobin (0.22 g, 88% of Theoretical yield). Reaction
monitoring by HPLC (% area): (Z)-Fluoxastrobin: 6.79% and
(E)-Fluoxastrobin: 88.84%. Isolated product HPLC purity (% area):
(Z)-Fluoxastrobin: 6.94% and (E)-Fluoxastrobin: 84.43%.
IR (cm−1,
KBr) 3066.28w, 2981.58w, 2939.36s, 2825.71w, 2500.61w, 1602.36s,
1572.76s, 1441.05s, 1297.05m, 1218.17m, 1116.52s, 1046.15m 1000.86w,
904.73s, 764.71w. 1H NMR (CDCl3, 400 MHz) δ 3.983
(s, 3H), 4.163-4.218 (t, 2H), 4.432-4.440 (t, J=3.2 Hz, 2H), 7.217-7.352
(m, 4H), 7.371-7.390 (m, 2H), 7.483-7.516 (m, 2H), 7.702-7.722 (d, J=8
Hz, 1H), 8.016 (s, 1H). MS (EI) m/z 459.1 (M+1); MS2 (EI) m/z 427.0,
382.9, 366.7, 340.0, 305.8, 246.1, 188.0. HPLC (Area %): 99.11%. M.P.
150-152° C.
Title: Fluoxastrobin
CAS Registry Number: 361377-29-9
CAS Name: (1E)-[2-[[6-(2-Chlorophenoxy)-5-fluoro-4-pyrimidinyl]oxy]phenyl](5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyloxime
Manufacturers' Codes: HEC-5725
Trademarks: Fandango (Bayer CropSci.)
Molecular Formula: C21H16ClFN4O5
Molecular Weight: 458.83
Percent Composition: C 54.97%, H 3.51%, Cl 7.73%, F 4.14%, N 12.21%, O 17.43%
Literature References:
Leaf-systemic broad-spectrum fungicide for use in cereal and food
crops; member of methoxyimiodihydro-dioxazines. Prepn (stereochem.
unspecified): U. Heinemann et al., DE 19602095; eidem, US 6103717 (1997, 2000 both to Bayer). Comprehensive description: S. Dutzmann et al., BCPC Conf. - Pests Dis. 2002, 365. Field trial in winter wheat seeds: I. Haeuser-Hahn et al., BCPC Int. Cong. - Crop Sci. Tech. 2003, 801. Series of articles on chemistry, biology, determn, and environmental fate: Pflanzenschutz-Nachr. Bayer (Engl. Ed.) 57, 299-449 (2004). Ecotoxicology: P. Breuer, ibid. 319.
Properties: White crystals with slight characteristic odor, mp 103-108°. bp 497° (est.). d420 1.422. Log P (octanol/water): 2.86 (20°). Vapor pressure at 20° (extrapolated): 6 ´ 10-10 Pa. Soly at 20° (g/l): n-heptane
0.04; 2-propanol 6.7; xylene 38.1; dichloromethane >250; in water
(mg/l): 2.56 (unbuffered); 2.43 (pH 4); 2.29 (pH 7); 2.27 (pH 9). LD50 in rats, bobwhite quail (mg/kg): >2500, >2000 orally; LC50 (96 hr) rainbow trout, bluegill sunfish, carp (mg/l): 0.44, 0.97, 0.57 (Breuer).
Melting point: mp 103-108°
Boiling point: bp 497° (est.)
Log P: Log P (octanol/water): 2.86 (20°)
Density: d420 1.422
Toxicity data: LD50 in rats, bobwhite quail (mg/kg): >2500, >2000 orally; LC50 (96 hr) rainbow trout, bluegill sunfish, carp (mg/l): 0.44, 0.97, 0.57 (Breuer)
Use: Agricultural fungicide.
MONITORING FLUORINATIONS......Selective direct fluorination for the synthesis of 2-fluoromalonate esters
Optimisation and real time reaction monitoring of the synthesis of 2-fluoromalonate esters by direct fluorination using fluorine gas is reported. An assessment of green metrics including atom economy and process mass intensity factors, demonstrates that the one-step selective direct fluorination process compares very favourably with established multistep processes for the synthesis of fluoromalonates.
Scheme 2 Synthetic routes to 2-fluoromalonate esters.
There are three realistic, low-cost
synthetic strategies available for the large scale manufacture of
diethyl 2-fluoromalonate ester (Scheme 2) which involve reaction of ethanol with hexafluoropropene (HFP), halogen exchange (Halex)and selective direct fluorination
processes. Other syntheses of fluoromalonate esters using electrophilic
fluorinating agents such as Selectfluor™ are possible, but are not
sufficiently commercially attractive to be considered for manufacture on
the large scale.
A growing number of patents
utilising fluoromalonate as a substrate for the synthesis of a range of
biologically active systems have been published
For example, Fluoxastrobin (Fandango®), a fungicide marketed by Bayer
CropScience that has achieved global annual sales of over €140 m since
its launch in 2005, and TAK-733, an anti-cancer drug candidate, employ 2-fluoromalonate esters as the key fluorinated starting material (Scheme 1).
| Scheme 1 2-Fluoromalonate esters used in the synthesis of Fluoxastrobin and TAK-733. | |||||||||||||||||||||
Before a comparison of the green metrics between
the three possible, economically viable large scale processes for the
synthesis of fluoromalonate esters (Scheme 2)
could be carried out, some primary goals for the optimisation of the
process were targeted: complete conversion of the starting material is
essential because it can be difficult to separate the starting material
from the desired monofluorinated product by simple distillation;
fluorine gas usage should be minimised because neutralisation of excess
reagent could potentially generate significant amounts of waste;
reduction in volumes of solvents used to reduce waste streams and
overall intensification of the fluorination process and replacement
and/or reduction of all environmentally harmful solvents used.
Conventional batch direct fluorination
reactions of malonate esters were carried out in glassware vessels by
introduction of fluorine gas, as a 10% or 20% mixture in nitrogen (v/v),
at a prescribed rate via a gas mass flow
controller into a solution of malonate ester and copper nitrate catalyst
in acetonitrile using equipment described previously.
To better understand the relationship between
fluorine gas introduction and rate of conversion, real time IR
spectroscopic monitoring of the reaction was chosen as the most suitable
technique. The use of the ReactIR technique was enabled by a sufficient difference in the carbonyl group stretching frequencies (1734 cm−1 for diethyl malonate and 1775 cm−1 for diethyl 2-fluoromalonate) and provided an in situ reaction profile (Fig. 1).
| Fig. 1 IR spectra of the fluorination reaction at 0% (light blue), 50% (dark blue) and 100% (red) conversions. | ||||||||
The real time reaction monitoring (Fig. 1 and 2)
revealed that the reaction begins instantly upon initiation of fluorine
introduction and the reaction conversion is directly proportional to
the amount of fluorine gas passed into the reaction vessel. When the
intensity of the fluoromalonate carbonyl peak (1775 cm−1) reached a maximum, the introduction of fluorine gas was stopped and the crude reaction mixture was analysed by 1H and 19F
NMR spectroscopy. Complete conversion of the starting material was
observed and diethyl fluoromalonate was formed with 93% selectivity
after introducing 1.1 equivalents of fluorine into the reaction mixture.
The small excess of fluorine explains the unexpectedly small amount of
difluorinated side products B and C
(4.5 and 2.5% respectively) which were the major impurities (6.5 and 9%
respectively) when larger excess of fluorine gas (1.8 eq.) was used.
The effect of concentration of fluorine in
nitrogen, reaction temperature, copper nitrate catalyst loading and
concentration of malonate substrate in acetonitrile were varied to
optimise the fluorination process (Table 1). Additionally, reactions described in Table 1
allowed an assessment of various factors that have a major influence on
the environmental impact of the process such as solvent usage, reaction
temperature and the amount and composition of waste generated. In each
case 20 mmol (3.20 g) of diethyl malonate was used as substrate and the
isolated mass balance of crude material obtained after work-up was
recorded along with the conversion of starting material and yield of
fluorinated products (Table 1).
Table 1 Fluorination of diethyl malonate ester using fluorine gas catalysed by Cu(NO3)2·2.5H2O
| Entry no. | T/°C | C malonate (mol L−1) | Catalyst (mol%) | F 2 in N2 (% v/v) | Conversion (1H NMR) | A/B/C ratio (19F NMR) | Isolated weight |
|---|---|---|---|---|---|---|---|
| 1 | 0–5 | 1.0 | 10 | 10 | 100% | 93.5/4.5/2 | 3.37 g |
| 2 | 0–5 | 1.5 | 10 | 10 | 100% | 94/4/2 | 3.30 g |
| 3 | 0–5 | 1.0 | 5 | 10 | 97% | 95/4/1 | 3.53 g |
| 4 | 0–5 | 1.0 | 2.5 | 10 | 82% | 95/4/1 | 3.51 g |
| 5 | RT | 1.0 | 10 | 10 | 56% | 97.5/1.5/1 | 3.33 g |
| 6 | 0–5 | 1.0 | 10 | 15 | 85% | 97.5/1.5/1 | 3.47 g |
| 7 | 0–5 | 1.0 | 10 | 20 | 100% | 94/3/3 | 3.50 g |
| 8 | 0–5 | 2.0 | 5 | 20 | 52% | 92/5/3 | 3.40 g |
In all cases, small quantities of side products were formed which were identified by 19F
NMR and these originate from two different processes:
3,3-difluoromalonate is produced from enolisation of diethyl
fluoromalonate which is much slower than enolisation of the diethyl
malonate substrate, while the fluoroethyl fluoromalonate is postulated
to form via an electrophilic process.
The data in Table 1
suggest that the concentration of the malonate ester substrate in
acetonitrile has no apparent effect on the outcome of the reaction
although solvent is required for these reactions because diethyl
malonate does not dissolve the catalyst. Additionally, the use of high
dielectric constant media, such as acetonitrile, have been found to be
beneficial for the control of selectivity of electrophilic direct
fluorination processes.
For convenience, a 1.5 M concentration of malonate in acetonitrile was
chosen as the optimal conditions which is approximately 5 mL solvent per
1 mL of diethyl malonate.
The concentration of fluorine gas, between
10–20% v/v in nitrogen, does not affect the selectivity of the reaction
and the quality of the product either, as exemplified by the product
mixtures obtained from reactions 1, 2 and 7 which have identical
compositions. In contrast, carrying out fluorination reactions at room
temperature rather than cooling the reaction mixture to 0–5 °C leads to
increased catalyst decomposition which results in an insoluble copper
species that on occasion blocked the fluorine gas inlet tube. In
addition, without cooling, the exothermic nature of this fluorination
reaction led to a slight reaction temperature increase (from 20 to 29 °C
in a small scale laboratory experiment) resulting in loss of some
solvent and some decomposition of the catalyst and product degradation.
Lowering the concentration of the copper
nitrate catalyst led to a significantly slower reaction as would be
expected and required the use of a larger excess of fluorine gas to
enable sufficiently high conversion. For example, the reaction proceeded
in the presence of only 2.5 mol% catalyst, but in this case 40% excess
fluorine was required to reach 100% conversion.
Typical literature work-up procedures for direct fluorination reactions
involve pouring the reaction mixture into 3 to 5 volumes of water and
extracting the resulting mixture three times with dichloromethane. The
combined organic fraction is typically washed with water, saturated
sodium bicarbonate solution and dried over sodium sulfate before
evaporation of the solvent to give the crude reaction product. We sought
to improve the work-up to enable recycling of the reaction solvent and
substitute the use of environmentally harmful dichloromethane in the
reaction work-up stage. Upon completion of fluorine gas addition,
acetonitrile was evaporated for reuse and then the residue was
partitioned between ethyl acetate and water, the organic phase was
washed with water, saturated Na2CO3
solution and saturated brine and dried prior to evaporation under
reduced pressure. Modification of the workup procedure in this manner
enables the recovery of acetonitrile and ethyl acetate and significantly
reduces the amount of aqueous waste generated. When direct reuse of the
recovered acetonitrile was attempted, a copper containing precipitate
was formed presumably because of the high HF content of the solvent
(0.63 M by titration). Therefore, before reuse of the solvent, HF must
be removed. Stirring the recovered reaction solvent with solid Na2CO3
lowered the acid content to an acceptable level (0.04 M) and when a
second fluorination reaction was carried out in the recovered,
neutralised acetonitrile, no change in the fluorination reaction profile
was observed.
Upon completion of these optimisation
studies, selective fluorination reactions of malonate esters were scaled
up to 40 g scale in the laboratory without experiencing any change in
product profile. Isolation of significant quantities of
monofluoromalonate A crude product (99% yield, 95%
purity) was achieved which could be used in the subsequent cyclisation
processes described below without further purification or, if high
purity material was required, could be purified by fractional vacuum
distillation (bp. 102–103 °C, 18 mbar) to produce 99% pure material in
77% yield.
Related malonate esters were also subjected
to direct fluorination using the optimised conditions established above.
In the case of di-tert-butyl malonate,
fluorination was carried out on 12 g scale. 100% conversion was reached
after the introduction of 1.2 equivalents of fluorine gas and the
desired product was isolated in 96% yield. The purity of the crude
product was higher than 97% by 1H and 19F
NMR spectroscopy without any further purification and as expected, the
only side product was the 2,2-difluorinated product (Scheme 3).
| Scheme 3 Fluorination of di-methyl and di-tert-butyl malonates. | ||
Diethyl fluoromalonate large scale fluorination
Diethyl malonate (40.0 g, 0.25 mol) and copper nitrate hydrate (Cu(NO3)2·2.5H2O; 5.81 g, 25 mmol) were dissolved in acetonitrile (200 mL) and placed in 500 mL fluorination vessel, cooled to 0–5 °C and stirred at 650 rpm using an overhead stirrer. After purging the system with N2 for 5 minutes, fluorine gas (20% v/v in N2, 80 mL min−1, 265 mmol) was introduced into the mixture for 6 hours and 30 minutes. The reactor was purged with nitrogen for 10 minutes, the solvent removed in vacuo and the residue partitioned between water (50 mL) and ethyl acetate (50 mL). The aqueous phase was extracted once more with ethyl acetate (50 mL) and the combined organic layers were washed with saturated NaHCO3 (25 mL) and brine (20 mL). After drying over sodium sulfate, the solvent was evaporated to leave diethyl 2-fluoromalonate (44.4 g, 99% yield, 95% purity) as a light yellow, transparent liquid. This crude product was distilled to afford high purity fluoromalonate (34.7 g, 77% yield, 99%+ purity) as a colourless liquid, bp. 102–103 °C (18 mbar), (lit.: 110–112 °C, 29 mbar), spectroscopic data as above.........N. Ishikawa, A. Takaoka and M. K. Ibrahim, J. Fluorine Chem., 1984, 25, 203–212 CrossRef CAS.
PAPER
REF
DOI: 10.1039/C5GC00402K
(Paper)
Green Chem., 2015, 17, 3000-3009
Fluorine gas for life science syntheses: green metrics to assess selective direct fluorination for the synthesis of 2-fluoromalonate esters†
Antal
Harsanyi
and
Graham
Sandford
*
Department of Chemistry, Durham University, South Road, Durham, DH1 3LE, UK. E-mail: graham.sandford@durham.ac.uk
Department of Chemistry, Durham University, South Road, Durham, DH1 3LE, UK. E-mail: graham.sandford@durham.ac.uk
Received
19th February 2015
, Accepted 17th March 2015
First published on the web 17th March 2015
Optimisation
and real time reaction monitoring of the synthesis of 2-fluoromalonate
esters by direct fluorination using fluorine gas is reported. An
assessment of green metrics including atom economy and process mass
intensity factors, demonstrates that the one-step selective direct
fluorination process compares very favourably with established multistep
processes for the synthesis of fluoromalonates.
Paper
Fluorine gas for life science syntheses: green metrics to assess selective direct fluorination for the synthesis of 2-fluoromalonate esters
Antal Harsanyia and
Graham Sandford*a
*Corresponding authors
aDepartment of Chemistry, Durham University, South Road, Durham, UK
E-mail: graham.sandford@durham.ac.uk
E-mail: graham.sandford@durham.ac.uk
Green Chem., 2015,17, 3000-3009
DOI: 10.1039/C5GC00402K
Subscribe to:
Comments (Atom)









