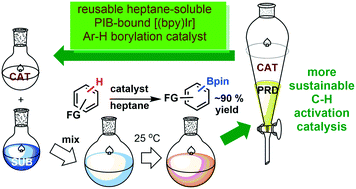
Highly active, separable and recyclable bipyridine iridium catalysts for C–H borylation reactions
Abstract
Iridium complexes generated from Ir(I) precursors and PIB oligomer functionalized bpy ligands efficiently catalyzed the reactions of arenes with bis(pinacolato)diboron under mild conditions to produce a variety of arylboronate compounds. The activity of this PIB bound homogeneous catalyst is similar to that of an original non-recyclable catalyst which allows it to be used under milder conditions than other reported recyclable catalysts. This oligomer-supported Ir catalyst was successfully recovered through biphasic extraction and reused for eight cycles without a loss of activity. Biphasic separation after the initial use of the catalyst led to an insignificant amount of iridium leaching from the catalyst to the product, and no iridium leaching from the catalyst was observed in the subsequent recycling runs. Arylboronate products obtained after extraction are sufficiently pure as observed by 1H and 13C-NMR spectroscopy that they do not require further purification.

Hind MAMLOUK, PhD
R&D in Organic Materials Chemistry Looking for a New Challenge

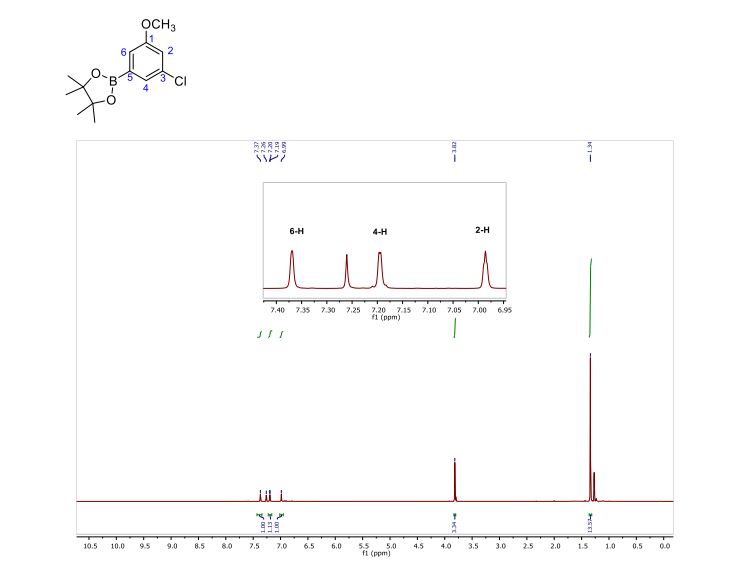
3-Chloro-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)anisole (5). Transparent oil. Yield: 87%.
1H NMR (600 MHz, CDCl3) δ 7.37 (s, 1H), 7.22 – 7.16 (m, 1H), 6.99 (s, 1H), 3.82 (s, 3H), 1.34 (s, 12H);
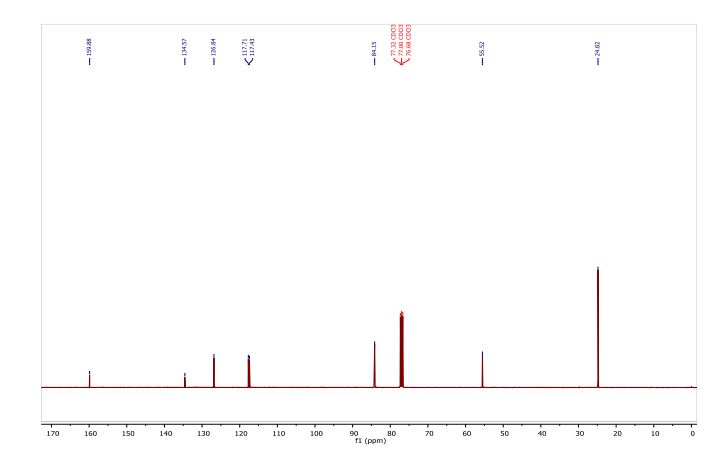
13C NMR (101 MHz, CDCl3) δ 159.88, 134.57, 126.84, 117.71, 117.43, 84.15, 55.52, 24.82.
GCMS: RT=14.55 min, M+ = 268.1 vs MW= 268.54 g.mol-1 .

Texas A&M University at Qatar

David Bergbreiter
Professor
Contact
Department of Chemistry
Texas A&M University
College Station, TX 77843-3255 P: 979-845-3437
F: 979-845-4719
bergbreiter@chem.tamu.edu
A common theme in our catalysis studies is exploring how soluble polymers can facilitate homogeneous catalysis. Homogeneous catalysts are ubiquitously used to prepare polymers, chemical intermediates, basic chemicals and pharmaceuticals. Such catalysts often use expensive or precious metals or expensive ligands or are used at relatively high catalyst loadings. The products often contain traces of these catalysts or ligands - traces that are undesirable for esthetic reasons or because of the potential toxicity of these impurities. Both the cost of these catalysts of these issues require catalyst/product separation - separations that often are inefficient and lead to chemical waste. These processes also use volatile organic solvents - solvents that have to be recovered and separated. Projects underway in our lab explore how soluble polymers can address each of these problems. Examples of past schemes that achieve this goal in a general way as highlighted in the Figure below.
We also use functional polymers to modify existing polymers. Ongoing projects involve molecular design of additives that can more efficiently modify polymers' physical properties. We also use functional polymers in covalent layer-by-layer assembly to surface polymers' surface chemistry. An example of this work is our use of 'smart' polymers that reversibly change from being water soluble cold to being insoluble and hydrophobic on heating. Such polymers' have been used by us to prepare 'smart' catalysts, 'smart' surfaces and membranes, and to probe fundamental chemistry underlying temperature and salt-dependent protein solvation.

Professor
Contact
Department of Chemistry
Texas A&M University
College Station, TX 77843-3255 P: 979-845-3437
F: 979-845-4719
bergbreiter@chem.tamu.edu
Current Activities
Our group explores new chemistry related to catalysis and polymer functionalization using the tools and precepts of synthetic organic chemistry to prepare functional oligomers or polymers that in turn are used to either effect catalysis in a greener, more environmentally benign way or to more efficiently functionalize polymers. Often this involves creatively combining the physiochemical properties of a polymer with the reactivity of a low molecular weight compound to form new materials with new functions. These green chemistry projects involve undamental research both in synthesis and catalysis but has practical aspects because of its relevance to practical problems.A common theme in our catalysis studies is exploring how soluble polymers can facilitate homogeneous catalysis. Homogeneous catalysts are ubiquitously used to prepare polymers, chemical intermediates, basic chemicals and pharmaceuticals. Such catalysts often use expensive or precious metals or expensive ligands or are used at relatively high catalyst loadings. The products often contain traces of these catalysts or ligands - traces that are undesirable for esthetic reasons or because of the potential toxicity of these impurities. Both the cost of these catalysts of these issues require catalyst/product separation - separations that often are inefficient and lead to chemical waste. These processes also use volatile organic solvents - solvents that have to be recovered and separated. Projects underway in our lab explore how soluble polymers can address each of these problems. Examples of past schemes that achieve this goal in a general way as highlighted in the Figure below.
We also use functional polymers to modify existing polymers. Ongoing projects involve molecular design of additives that can more efficiently modify polymers' physical properties. We also use functional polymers in covalent layer-by-layer assembly to surface polymers' surface chemistry. An example of this work is our use of 'smart' polymers that reversibly change from being water soluble cold to being insoluble and hydrophobic on heating. Such polymers' have been used by us to prepare 'smart' catalysts, 'smart' surfaces and membranes, and to probe fundamental chemistry underlying temperature and salt-dependent protein solvation.

Jakkrit Suriboot
Research Assistant at Texas A&M University

Dr. Praveen Kumar
Title: Research Assistant Professor
Education: M.S., I.I.T. Roorkee
Ph.D., Panjab University Chandigarh (2008)
Visiting Fellow (w/ Prof. G. G. Balint-Kurti), Bristol University, UK
Postdoctoral Research Associate (w/ Prof. Svetlana Malinovskaya), Stevens Institute of Technology, Hoboken, NJ
Senior Postdoctoral Research Associate (w/ Prof. Seogjoo Jang), Queens College of CUNY, NY
Office: Chemistry 010
Phone: 806-742-3124
Email: praveen.kumar@ttu.edu
Ph.D., Panjab University Chandigarh (2008)
Visiting Fellow (w/ Prof. G. G. Balint-Kurti), Bristol University, UK
Postdoctoral Research Associate (w/ Prof. Svetlana Malinovskaya), Stevens Institute of Technology, Hoboken, NJ
Senior Postdoctoral Research Associate (w/ Prof. Seogjoo Jang), Queens College of CUNY, NY
Office: Chemistry 010
Phone: 806-742-3124
Email: praveen.kumar@ttu.edu
///////////////
“ORG SYN INT” CATERS TO EDUCATION GLOBALLY, No commercial exploits are done or advertisements added by me. This is a compilation for educational purposes only. P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent



No comments:
Post a Comment