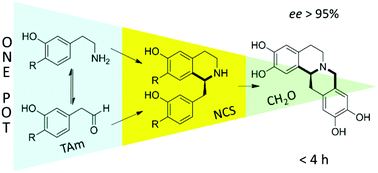
One-pot triangular chemoenzymatic cascades for the syntheses of chiral alkaloids from dopamine
Green Chem., 2015, 17,852-855
DOI: 10.1039/C4GC02325K, Communication
DOI: 10.1039/C4GC02325K, Communication
*Corresponding authors
aDepartment of Biochemical Engineering, University College London, Bernard Katz Building, Gordon Street, London, UK
E-mail: j.ward@ucl.ac.uk
bDepartment of Chemistry, University College London, Christopher-Ingold Building, 20 Gordon Street, London, UK
E-mail: h.c.hailes@ucl.ac.uk
Green Chem., 2015,17, 852-855
DOI: 10.1039/C4GC02325K

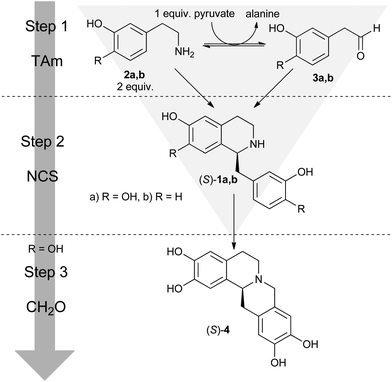 | ||
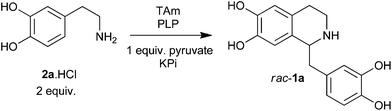
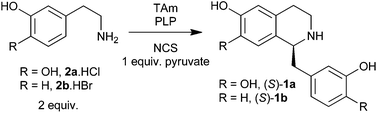
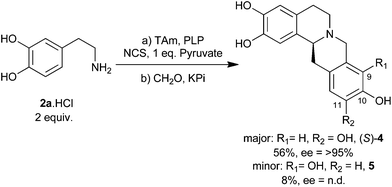
| Scheme 1 Overview of the biocatalytic and non-enzymatic cascades presented in this work, including the ‘triangular’ cascade. | ||

Prof Helen Hailes

Appointment
- Professor of Chemical Biology
- Dept of Chemistry
- Faculty of Maths & Physical Sciences
Research Themes
Research Summary
Research activity in our group is focused on the use of synthetic organic chemistry to probe and solve biological problems. Current projects include the use of water as a reaction solvent and the use of catalytic and biocatalytic synthetic strategies. Also novel kinase inhibitors, cytosine-based hydrogen-bonding polymers and new lipid design for use in a ternary gene delivery vector.
Academic Background
| 1991 | PhD | Doctor of Philosophy – Organic Chemistry | University of Cambridge |
| 1987 | BA | Bachelor of Arts – Chemistry and Metallurgy | University of Cambridge |
publications..total 146
9 Research Activities
Status

No comments:
Post a Comment