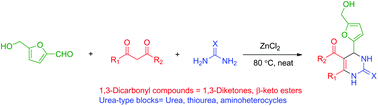
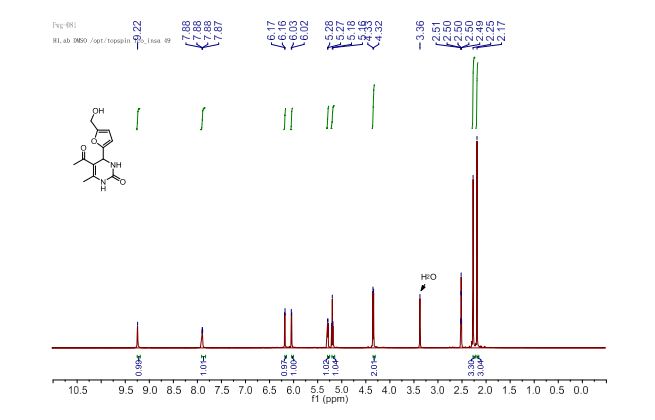
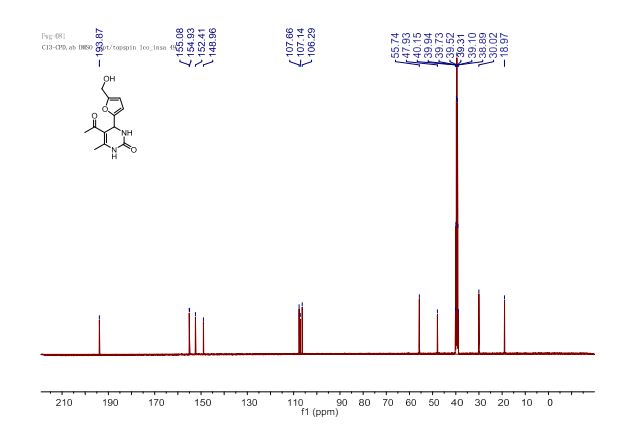
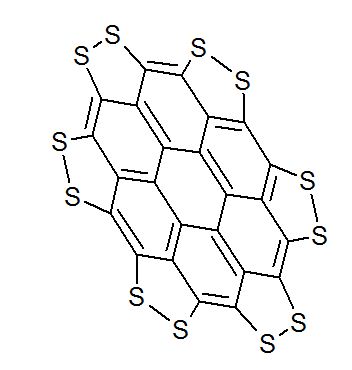
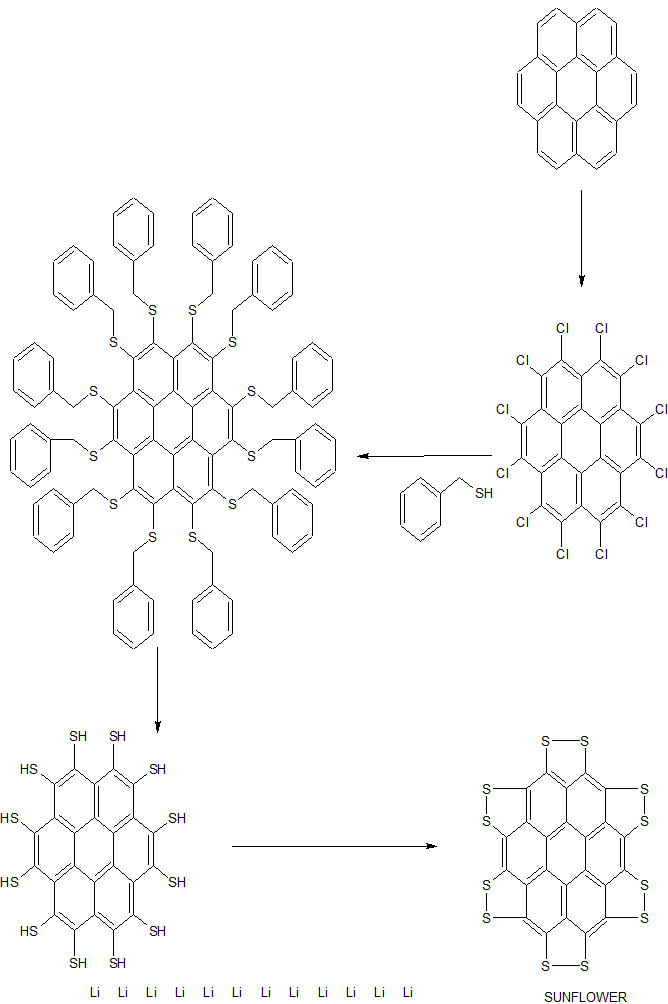
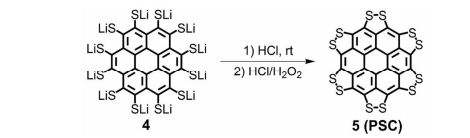
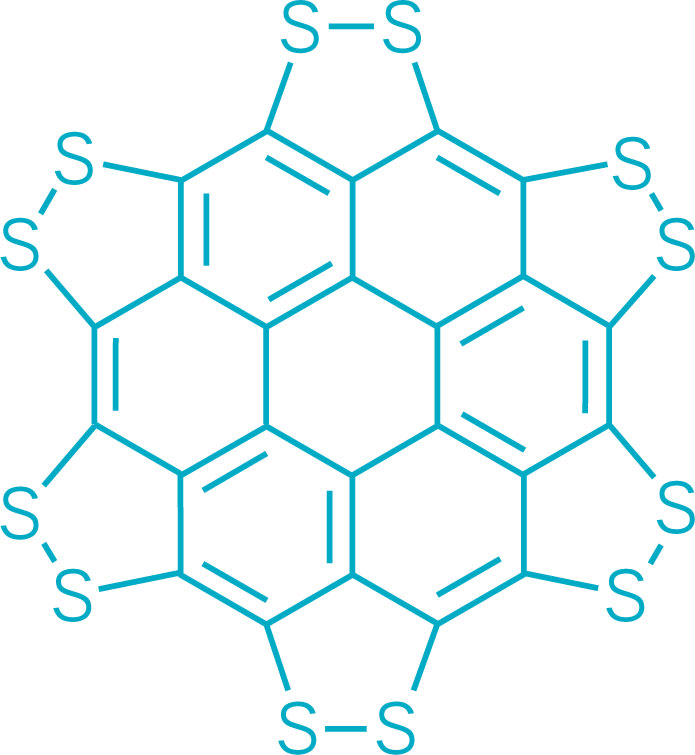
J. Org. Chem. 2014, 79 (5), 2331-2336 ; DOI: 10.1021/jo500131h
2-Phenyl-1,4-naphthoquinone 3a. 22 Following the general procedure, the crude product was purified over a silica gel column using a hexane/EtOAc mobile phase (9:1) to give a yellowish solid (140 mg, 60% yield): mp 107−109 °C; 1 H NMR (300 MHz, CDCl3) δ 8.18−8.10 (m, 2H), 7.86−7.75 (m, 2H), 7.56−7.48 (m, 5H), 7.07 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 185.2, 185.1, 148.1, 135.2, 133.9 2-Phenyl-1,4-naphthoquinone 3a. 22 Following the general procedure, the crude product was purified over a silica gel column using a hexane/EtOAc mobile phase (9:1) to give a yellowish solid (140 mg, 60% yield): mp 107−109 °C; 1 H NMR (300 MHz, CDCl3) δ 8.18−8.10 (m, 2H), 7.86−7.75 (m, 2H), 7.56−7.48 (m, 5H), 7.07 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 185.2, 185.1, 148.1, 135.2, 133.9
2-Phenyl-1,4-naphthoquinone 3a. 22 Following the general procedure, the crude product was purified over a silica gel column using a hexane/EtOAc mobile phase (9:1) to give a yellowish solid (140 mg, 60% yield): mp 107−109 °C; 1 H NMR (300 MHz, CDCl3) δ 8.18−8.10 (m, 2H), 7.86−7.75 (m, 2H), 7.56−7.48 (m, 5H), 7.07 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 185.2, 185.1, 148.1, 135.2, 133.9 2-Phenyl-1,4-naphthoquinone 3a. 22 Following the general procedure, the crude product was purified over a silica gel column using a hexane/EtOAc mobile phase (9:1) to give a yellowish solid (140 mg, 60% yield): mp 107−109 °C; 1 H NMR (300 MHz, CDCl3) δ 8.18−8.10 (m, 2H), 7.86−7.75 (m, 2H), 7.56−7.48 (m, 5H), 7.07 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 185.2, 185.1, 148.1, 135.2, 133.9
CONCLUSION
In conclusion, we demonstrated a
new method for radical mediated arylation of naphthoquinones using the
combination of IBX with arylhydrazines. It does not require necessity of
transition metal catalysis and prefunctionalization on naphthoquinone moiety.
The reactions occurred under mild conditions in open atmosphere. Further, both
2-hydroxy and 2-amino groups were found to be tolerated under optimized
reaction conditions. This fact could be attributed to rapid reaction between
IBX and phenyl hydrazine. A postulated radical mediated mechanism was supported
by radical trapping experiments. Developed protocols were successfully extended
towards an effective, short and high yielding synthesis of benzocarbazoledione.
IBX mediated developed protocols for arylation could open a new field in
quinone chemistry as well as in the development of new procedures for arylation
of electron deficient molecules in near future
Aryl-Free Radical-Mediated Oxidative Arylation of Naphthoquinones Using o-Iodoxybenzoic Acid and Phenylhydrazines and Its Application toward the Synthesis of Benzocarbazoledione
Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, Matunga, Mumbai 400019, India
J. Org. Chem., 2014, 79 (5), pp 2331–2336
DOI: 10.1021/jo500131h
Publication Date (Web): February 10, 2014
Copyright © 2014 American Chemical Society
*E-mail: kgap@rediffmail.com; kg.akamanchi@ictmumbai.edu.in.
Abstract
Oxidative arylation of naphthoquinones has been developed through combination of o-iodoxybenzoic acid with arylhydrazines under mild conditions at open atmosphere. Arylated naphthoquinones with different electronic properties were obtained in moderate to good yields. The postulated radical mediated mechanism is supported by radical trapping experiments. Developed protocol for direct arylation of naphthoquinones has been extended toward short, high yielding, and an effective synthesis of antitumor–antibiotic precursor such as benzocarbazoledione.
ABOUT GUEST BLOGGER

Dr. Pravin C. Patil
Postdoctoral Research Associate at University of Louisville
Dr. Pravin C Patil completed his B.Sc. (Chemistry) at ASC College Chopda (Jalgaon, Maharashtra, India) in 2001 and M.Sc. (Organic Chemistry) at SSVPS’S Science College Dhule in North Maharashtra University (Jalgaon, Maharashtra, India) in year 2003. After M.Sc. degree he was accepted for summer internship training program at Bhabha Atomic Research Center (BARC, Mumbai) in the laboratory of Prof. Subrata Chattopadhyay in Bio-organic Division. In 2003, Dr. Pravin joined to API Pharmaceutical bulk drug company, RPG Life Science (Navi Mumbai, Maharashtra, India) and worked there for two years. In 2005, he enrolled into Ph.D. (Chemistry) program at Institute of Chemical Technology (ICT), Matunga, Mumbai, aharashtra, under the supervision of Prof. K. G. Akamanchi in the department of Pharmaceutical Sciences and Technology.
After finishing Ph.D. in 2010, he joined to Pune (Maharashtra, India) based pharmaceutical industry, Lupin Research Park (LRP) in the department of process development. After spending two years at Lupin as a Research Scientist, he got an opportunity in June 2012 to pursue Postdoctoral studies at Hope College, Holland, MI, USA under the supervision of Prof. Moses Lee. During year 2012-13 he worked on total synthesis of achiral anticancer molecules Duocarmycin and its analogs. In 2014, he joined to Prof. Frederick Luzzio at the Department for Chemistry, University of Louisville, Louisville, KY, USA to pursue postdoctoral studies on NIH sponsored project “ Structure based design and synthesis of Peptidomimetics targeting P. gingivalis.
During his research experience, he has authored 23 international publications in peer reviewed journals and inventor for 4 patents.
Prof K. G. Akamanchi
//////////////