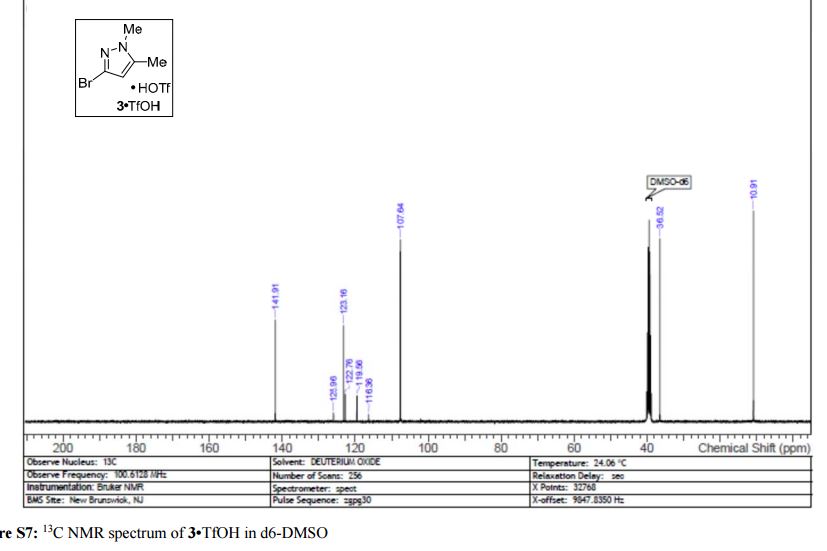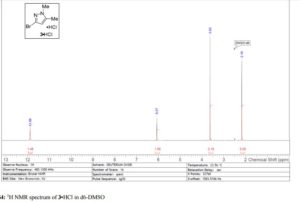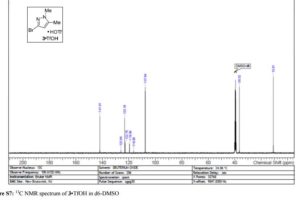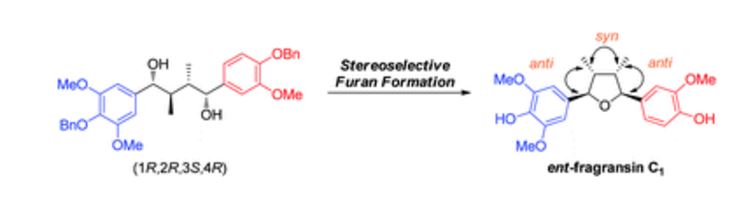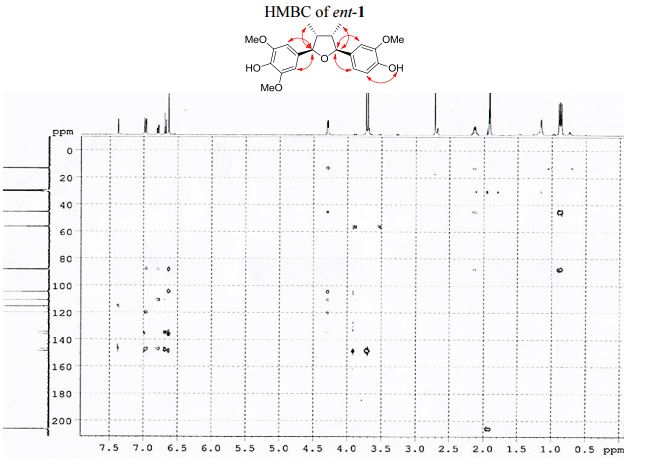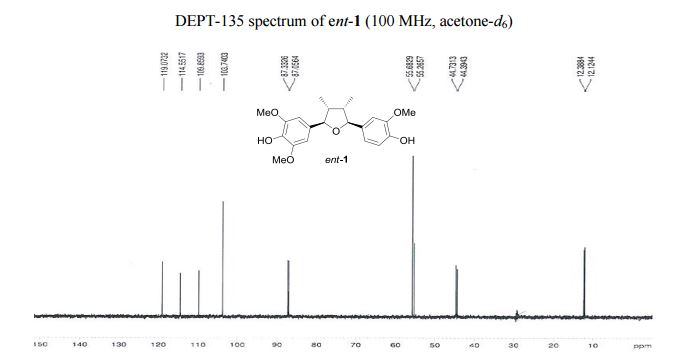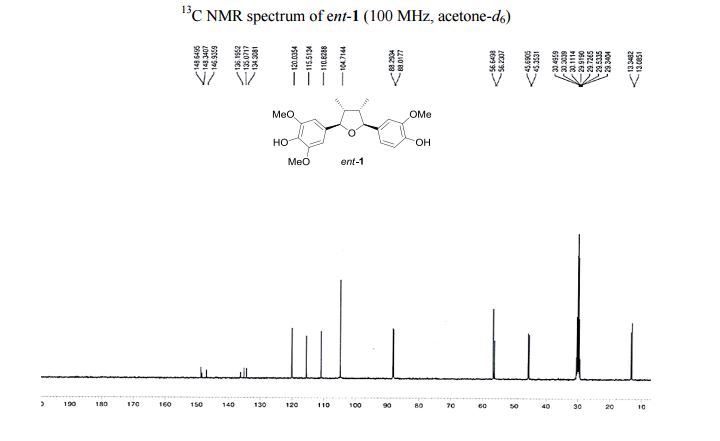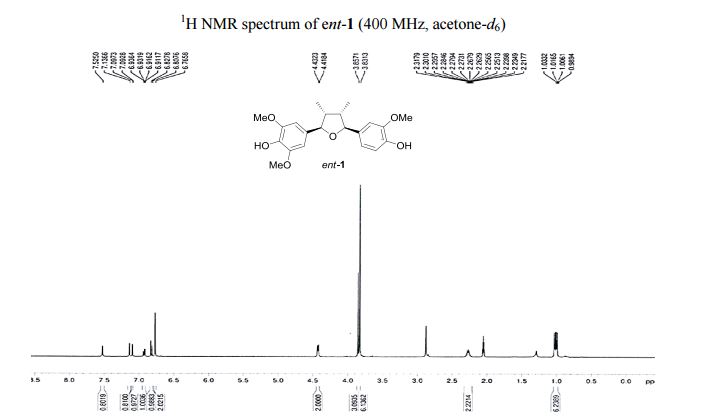
Iron-catalyzed dehydrogenation reactions and their applications in sustainable energy and catalysis
Catal. Sci. Technol., 2017, Advance Article
DOI: 10.1039/C7CY00879A, Minireview
DOI: 10.1039/C7CY00879A, Minireview
Catalysis Science & Technology
Iron-catalyzed dehydrogenation reactions and their applications in sustainable energy and catalysis
Abstract
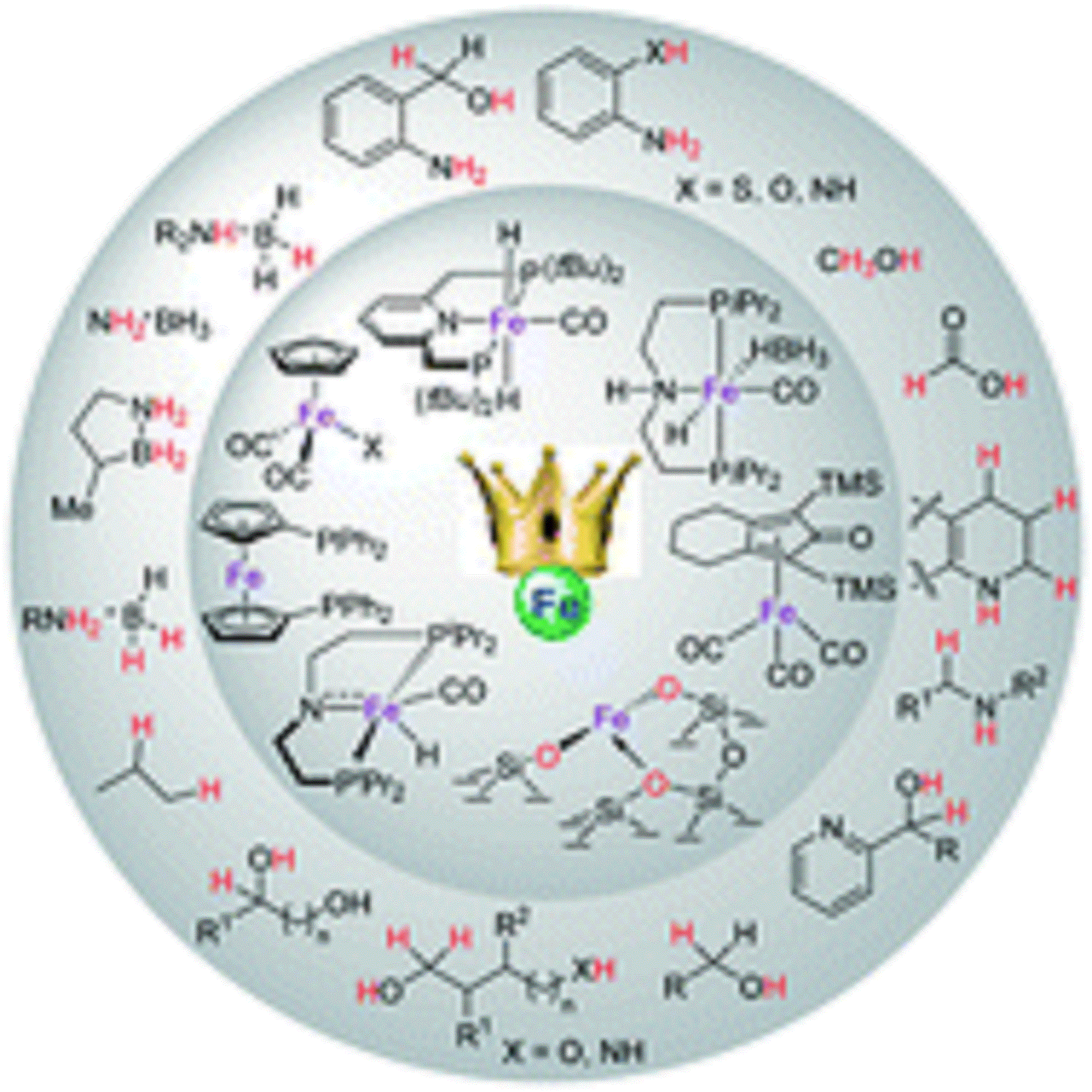
Inspired by nature, chemists have designed new catalysts in the pursuit of selective bond activation and chemical transformations. Emergent biological systems often use earth-abundant first-row transition elements as catalytically active sites to facilitate specific and highly selective chemical processes. The design of a new catalytic system based on abundant and inexpensive catalysts, particularly the iron-based catalysts, for fundamentally significant synthetic transformations under environmentally benign conditions is an important paradigm in chemical synthesis. In recent times, iron-based catalytic systems have shown unprecedented reactivity in the acceptorless dehydrogenation reactions of feedstock chemicals, with the liberation of molecular hydrogen as the by-product, and have enabled greener chemical synthetic methods and alternative energy storage systems. Indeed, it has been demonstrated that the proper design of iron catalysts by judiciously choosing ligands, can aid in the development of new sustainable energy storage systems and catalysis. This tutorial review focuses on the recent development of iron-based dehydrogenation reactions of fundamentally important feedstock, as a route to sustainable chemical synthesis and energy storage applications. The emerging area of the iron-based dehydrogenation strategy provides an opportunity to make industrially applicable, cost-effective and environmentally benign catalytic systems

Dr.Ekambaram Balaraman
Catalysis Division
CSIR-National Chemical Laboratory
Catalysis Division
CSIR-National Chemical Laboratory

//////////////