Imine Reduction
Using Iron Catalysts
Abnormal-NHC-Fe(0) complex outperforms noble metal catalysts
Read more
http://www.chemistryviews.org/details/news/9749451/Imine_Reduction_Using_Iron_Catalysts.html?elq_mid=11741&elq_cid=1558306
A Highly Efficient Base-Metal Catalyst: Chemoselective Reduction of Imines to Amines Using An Abnormal-NHC–Fe(0) Complex
† Department of Chemical Sciences, Indian Institute of Science Education and Research Kolkata, Mohanpur 741246, India
‡ Department of Chemical Sciences, Indian Institute of Science Education and Research Mohali, SAS Nagar 140306, India
Organometallics, Article ASAP
DOI: 10.1021/acs.organomet.6b00478
P
*E-mail for S.K.M.: swadhin.mandal@iiserkol.ac.in.
A base-metal, Fe(0)-catalyzed hydrosilylation of imines to obtain amines is reported here which outperforms its noble-metal congeners with the highest TON of 17000. The catalyst, (aNHC)Fe(CO)4, works under very mild conditions, with extremely low catalyst loading (down to 0.005 mol %), and exhibits excellent chemoselectivity. The facile nature of the imine reduction under mild conditions has been further demonstrated by reducing imines towards expensive commercial amines and biologically important N-alkylated sugars, which are difficult to achieve otherwise. A mechanistic pathway and the source of chemoselectivity for imine hydrosilylation have been proposed on the basis of the well-defined catalyst and isolable intermediates along the catalytic cycle.
Swadhin K. Mandal

Professional Recognitions
Swadhin K. Mandal
Swadhin Mandal
Associate Professor
Dept: Chemical Sciences (DCS) E-mail: swadhin.mandal [at] iiserkol.ac.in Personal homepage: Click Here |
Research Interest:
Organometallic chemistry and its application in catalysis, new drug development and material chemistry
Academic Background:
- BSc (Chemistry), University of Kalyani, 1993, Secured 3rd Rank in University
- MSc (Chemistry), University of Kalyani, 1996, Secured first rank in University
- PhD (Chemistry), Indian Institute of Science Bangalore, 2002
Positions:
- Postdoctoral Fellow, University of California, Riverside (2002 - 2005)
- Alexander von Humboldt Fellow, University of Goettingen (2006 - 2007)
- Assistant Professor, IISER Kolkata (2007 - 2013)
- Associate Professor, IISER Kolkata (2013 - 2014)
- Associate Professor, IISER Kolkata ( - )
Awards and Honors:
- Alexander von Humboldt Fellowship from Alexander von Humboldt Foundation (2005)
- YIM Boston Young Scientist Award from YIM Boston at MIT, USA (2012)
Selected Publications
Professional Recognitions
Selected Publications:

Gonela Vijay Kumar, SRF
Research Interest:
Development of Nucleophilic Boron Compounds and its Reactivity.
Email - gonela.vijaykumar@gmail.com

Pradip Kumar Hota, SRF
Research Interest:
Transition Metal Mediated C-C Coupling Reactions.
Email - pradip.hota87@gmail.com
- Raman, Karthik V; Kamerbeek, Alexander M.; Mukherjee, Arup; Atodiresei, Nicolae; Sen, Tamal K; Lazic, Predrag; Caciuc, Vasile; Michel, Reent; Stalke, Dietmar; Mandal, Swadhin K; Bluegel, Stephan; Muenzenberg, Markus and Moodera, Jagadeesh. 2013. "Interface-engineered templates for molecular spin memory devices." Nature, 493, 509-513
- Sen, Tamal K; Mukherjee, Arup; Modak, Arghya; Mandal, Swadhin K and Koley, Debasis. 2013. "Substitution Effect on Phenalenyl Backbone in the Rate of Organozinc Catalyzed ROP of Cyclic Esters." Dalton Trans., 42, 1893-1904
- Mukherjee, Arup; Sen, Tamal K.; Ghorai, Pradip Kr.; Samuel, Prinson P.; Schulzke, Carola and Mandal, Swadhin K. 2012. "Phenalenyl-Based Organozinc Catalysts for Intramolecular Hydroamination Reactions: A Combined Catalytic, Kinetic, and Mechanistic Investigation of the Catalytic Cycle." Chemistry -A European Journal, 18, 10530-545
Gonela Vijay Kumar, SRF
Research Interest:
Development of Nucleophilic Boron Compounds and its Reactivity.
Email - gonela.vijaykumar@gmail.com
Pradip Kumar Hota, SRF
Research Interest:
Transition Metal Mediated C-C Coupling Reactions.
Email - pradip.hota87@gmail.com

















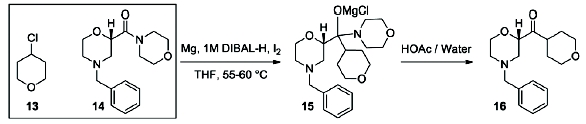
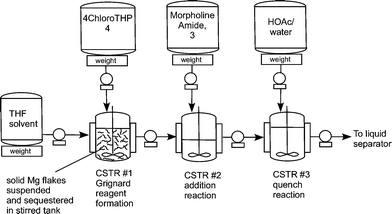
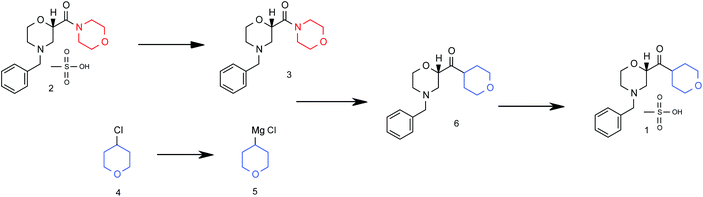
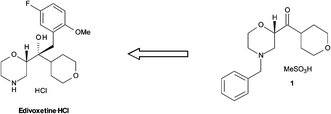
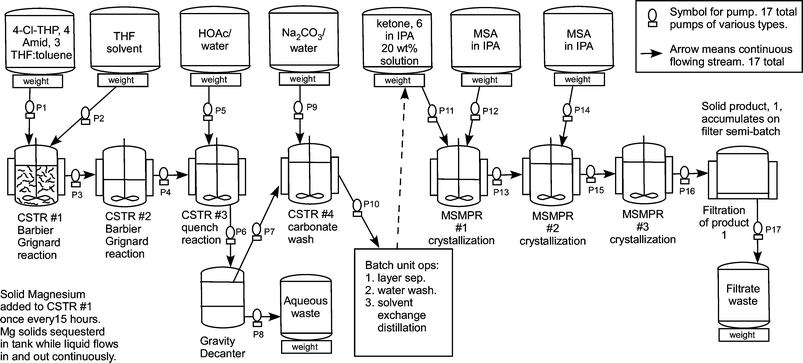 Flow diagram for the whole continuous process from amide 3 to product 1.
Flow diagram for the whole continuous process from amide 3 to product 1.