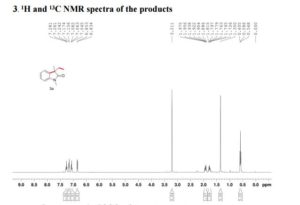
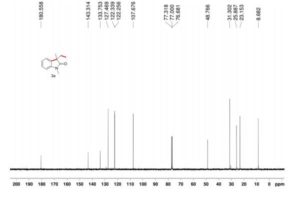
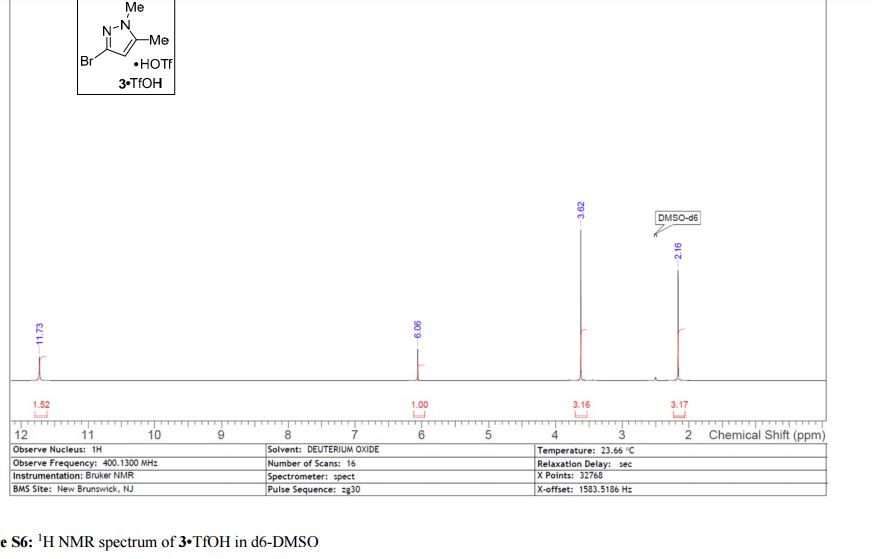
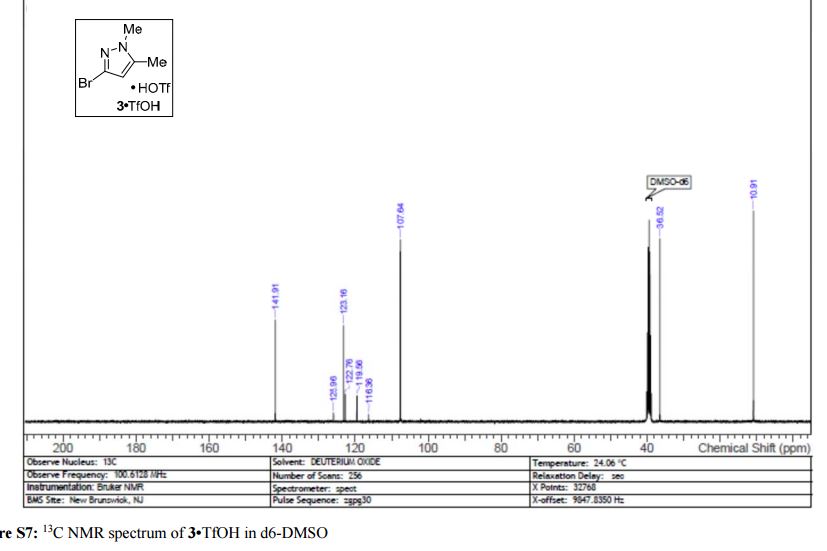
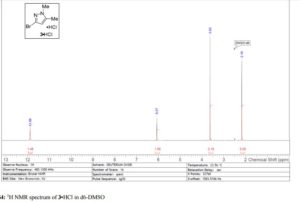
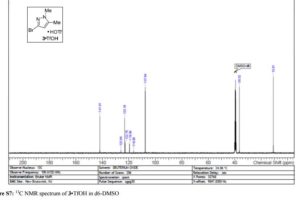
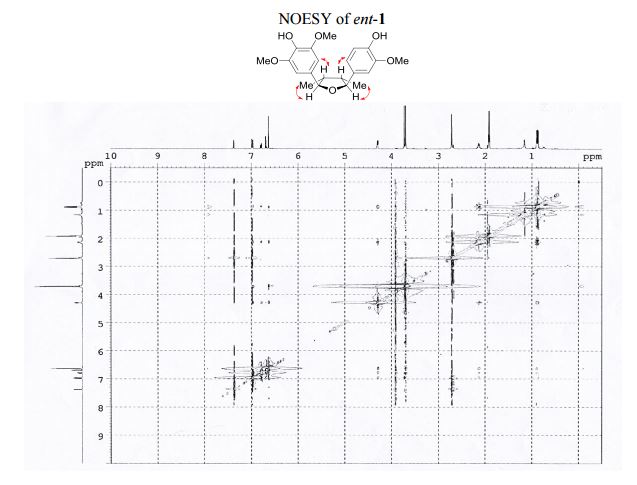
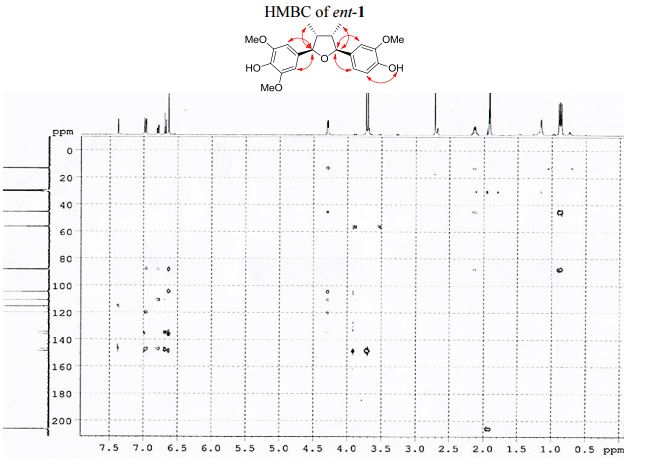
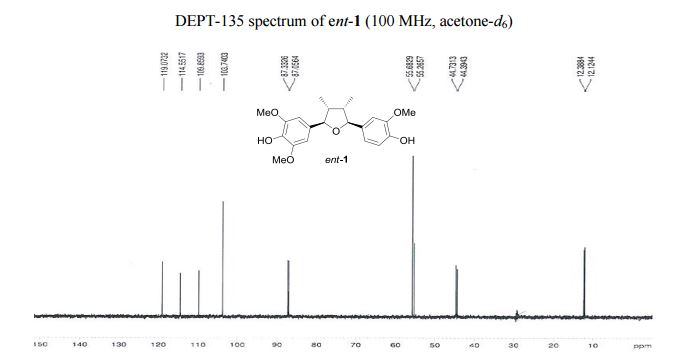
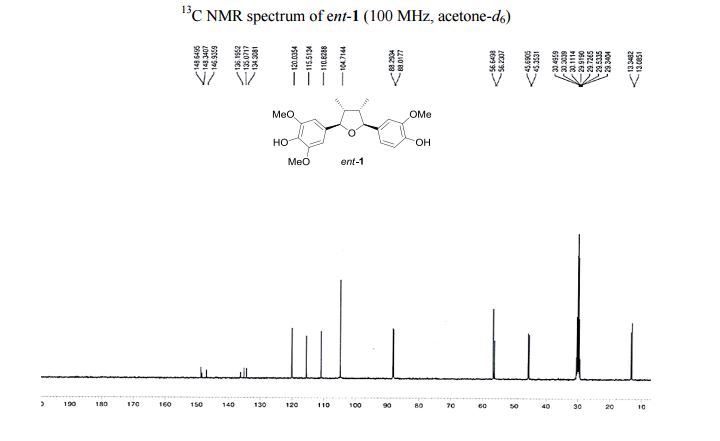
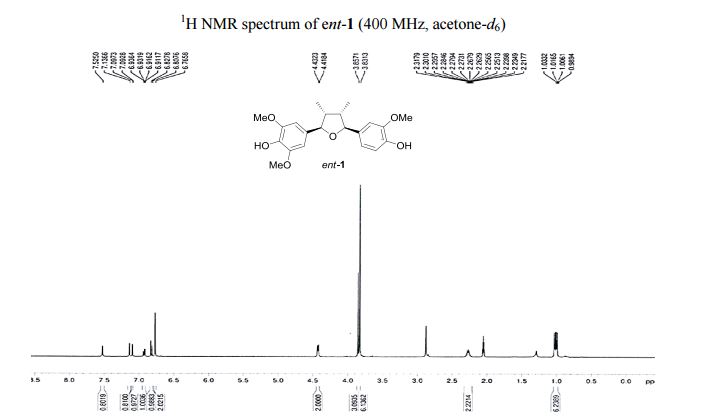
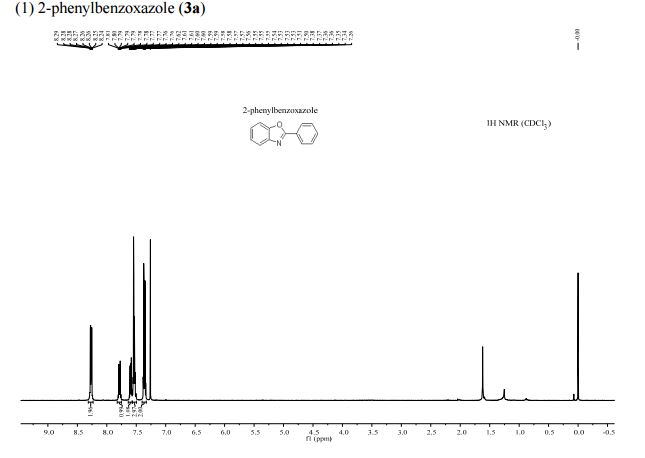
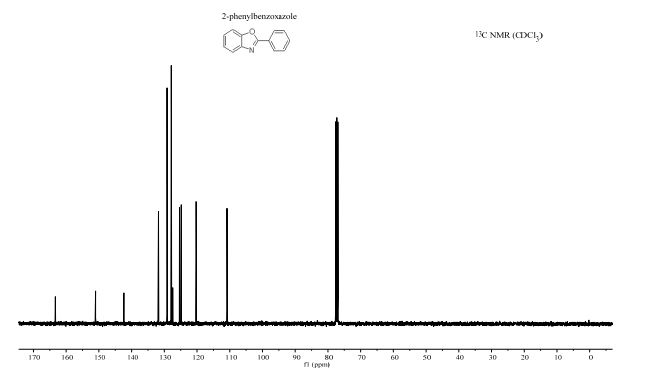
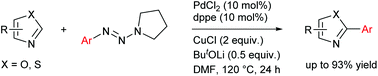
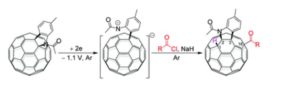
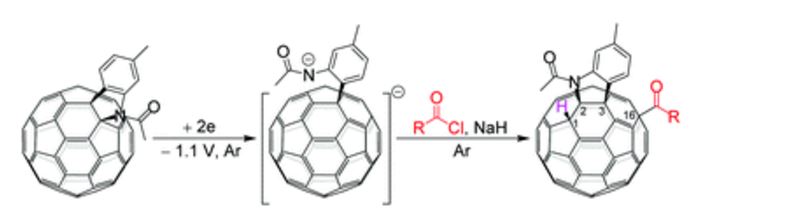
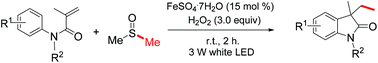Visible-light-induced and iron-catalyzed methylation of N-arylacrylamides with dimethyl sulphoxide: a convenient access to 3-ethyl-3-methyl oxindoles
Org. Biomol. Chem., 2017, Advance Article
DOI: 10.1039/C7OB00779E, Paper
DOI: 10.1039/C7OB00779E, Paper
Zuguang Xie, Pinhua Li, Yu Hu, Ning Xu, Lei Wang
An efficient synthesis of 3-ethyl-3-methyl oxindoles by visible-light promoted and iron-catalyzed difunctionalization of N-arylacrylamides with dimethyl sulphoxide was developed
An efficient synthesis of 3-ethyl-3-methyl oxindoles by visible-light promoted and iron-catalyzed difunctionalization of N-arylacrylamides with dimethyl sulphoxide was developed
Visible-light-induced and iron-catalyzed methylation of N-arylacrylamides with dimethyl sulphoxide: a convenient access to 3-ethyl-3-methyl oxindoles
Abstract
A visible-light-induced and iron-catalyzed methylation of arylacrylamides by dimethyl sulphoxide (DMSO) is achieved, leading to 3-ethyl-3-methyl indolin-2-ones in high yields. This reaction tolerates a series of functional groups, such as methoxy, trifluoromethyl, cyano, nitro, acetyl and ethyloxy carbonyl groups. The visible-light promoted radical methylation and arylation of the alkenyl group are involved in this reaction.
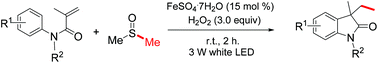
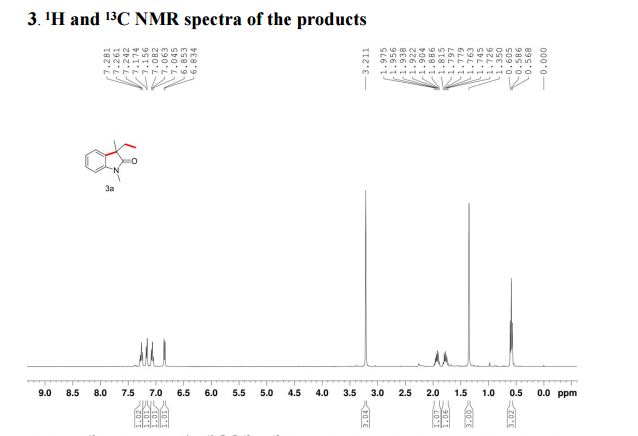
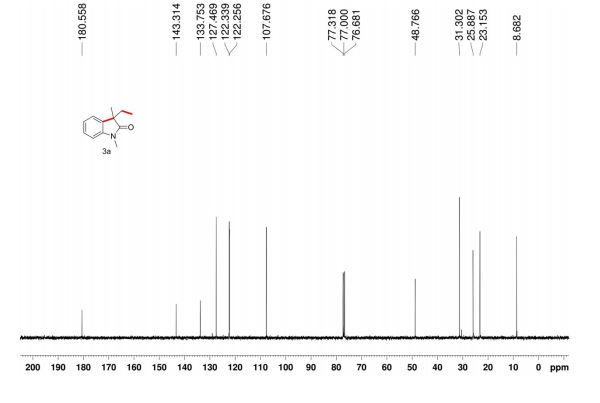
//////