React. Chem. Eng., 2016, Advance Article
DOI: 10.1039/C6RE00059B, Paper
DOI: 10.1039/C6RE00059B, Paper
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
Nicholas Holmes, Geoffrey R. Akien, A. John Blacker, Robert L. Woodward, Rebecca E. Meadows, Richard A. Bourne
Self-optimising flow reactors combine online analysis with evolutionary feedback algorithms to rapidly achieve optimum conditions.
Self-optimising flow reactors combine online analysis with evolutionary feedback algorithms to rapidly achieve optimum conditions.
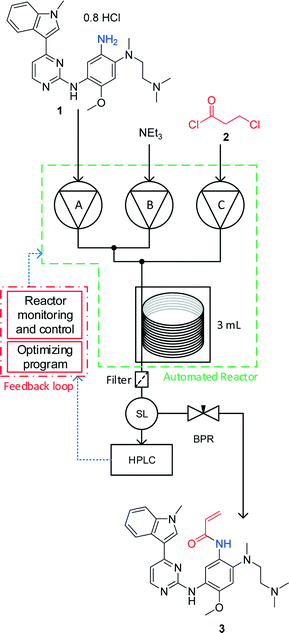
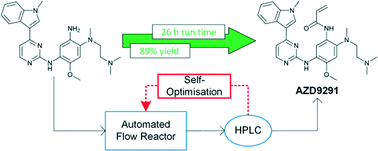
Self-optimisation of the final stage in the synthesis of EGFR kinase inhibitor AZD9291 using an automated flow reactor
Self-optimising flow reactors combine online analysis with evolutionary feedback algorithms to rapidly achieve optimum conditions. This technique has been applied to the final bond-forming step in the synthesis of AZD9291, an irreversible epidermal growth factor receptor kinase inhibitor developed by AstraZeneca. A four parameter optimisation of a telescoped amide coupling followed by an elimination reaction was achieved using at-line high performance liquid chromatography. Optimisations were initially carried out on a model compound (2,4-dimethoxyaniline) and the data used to track the formation of various impurities and ultimately propose a mechanism for their formation. Our protocol could then be applied to the optimisation of the 2-step telescoped reaction to synthesise AZD9291 in 89% yield.
Paper
Self-optimisation of the final stage in the synthesis of EGFR kinase inhibitor AZD9291 using an automated flow reactor
*Corresponding authors
aInstitute of Process Research and Development, School of Chemistry, University of Leeds, Leeds, UK
E-mail: r.a.bourne@leeds.ac.uk
E-mail: r.a.bourne@leeds.ac.uk
bDepartment of Chemistry, Faraday Building, Lancaster University, Lancaster, UK
cSchool of Chemical and Process Engineering, University of Leeds, Leeds, UK
dAstraZeneca Pharmaceutical Development, Silk Road Business Park, Macclesfield, UK
React. Chem. Eng., 2016, Advance Article
DOI: 10.1039/C6RE00059B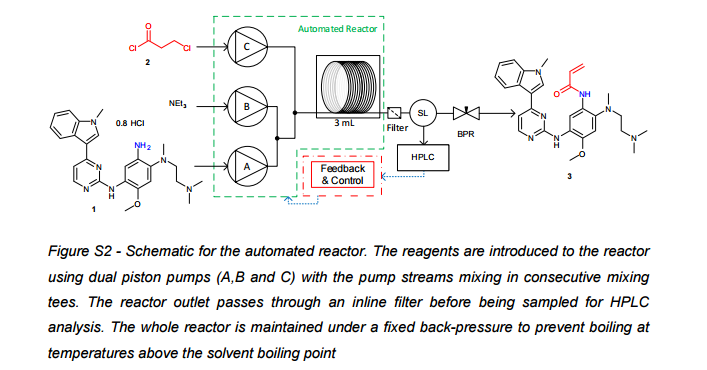
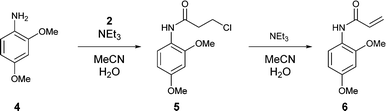
| Scheme 1 Synthesis of the model acrylamide 6 via the β-chloroamide 5 intermediate. |
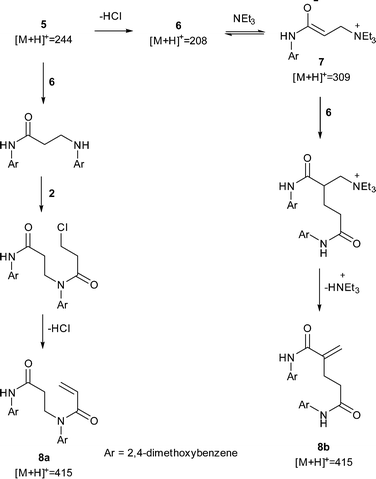
| Scheme 2 Proposed mechanisms to dimers 8a and 8b. The observation of a peak corresponding to 7suggested a Rauhut–Currier mechanism to 8b but subsequent LC-MS-MS analysis showed the major dimer to most likely be 8a. All observed peaks from offline LC-MS are displayed. |
///////Self-optimisation, synthesis, EGFR kinase inhibitor, AZD9291, automated flow reactor