A catalytic aldol condensation system enables one pot conversion of biomass saccharides to biofuel intermediates
Abstract
Producing bio-intermediates from lignocellulosic biomass with minimal process steps has a far-reaching impact on the biofuel industry. We studied the metal chloride catalyzed aldol condensation of furfural with acetone under conditions compatible with the upstream polysaccharide conversions to furfurals. In situ far infrared spectroscopy (FIR) was applied to guide the screening of aldol condensation catalysts based on the distinguishing characteristics of metal chlorides in their coordination chemistries with carbonyl-containing compounds. NiCl2, CoCl2, CrCl3, VCl3, FeCl3, and CuCl2 were selected as the potential catalysts in this study. The FIR results further helped to rationalize the excellent catalytic performance of VCl3 in furfural condensation with acetone, with 94.7% yield of biofuel intermediates (C8, C13) in 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) solvent. Remarkably, addition of ethanol facilitated the acetal pathway of the condensation reaction, which dramatically increased the desired product selectivity over the furfural pathway. Most significantly, we demonstrate for the first time that VCl3 catalyzed aldol condensation in acidic medium is fully compatible with upstream polysaccharide hydrolysis to monosaccharide and the subsequent monosaccharide isomerization and dehydration to furfurals. Our preliminary results showed that a 44% yield of biofuel intermediates (C8, C13) can be obtained in one-pot conversion of xylose catalyzed by paired metal chlorides, CrCl2 and VCl3. A number of prior works have shown that the biofuel intermediates derived from the one-pot reaction of this work can be readily hydrogenated to biofuels.
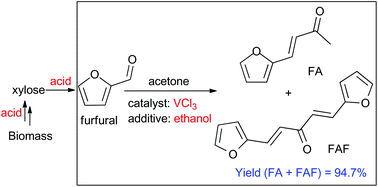
1,5-bis-(2-furanyl)-1,4-pentadien-3-one (FAF)
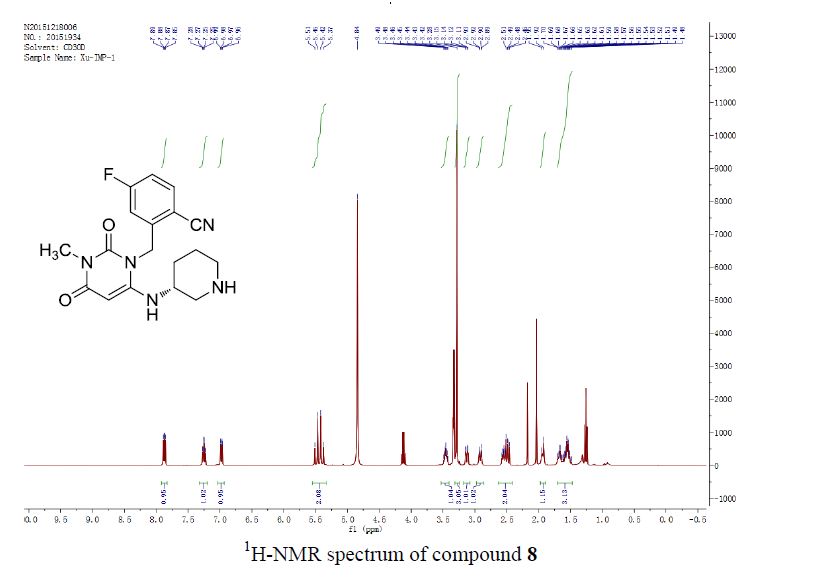
FAF is a yellow solid.1H NMR (400 MHz, CDCl3, TMS) δ 7.51 – 7.46 (m, 4H), 6.92 (d, J = 15.6 Hz, 2H), 6.69 (d, J = 3.4 Hz, 2H), 6.50 – 6.49 (m, 2H);13C NMR (100 MHz, CDCl3) δ 188.1, 151.6, 144.9, 129.2, 123.2, 115.8, 112.6




 gene.com
gene.com